IMPROVING THE QUALITY OF PETROLEUM PRODUCTS
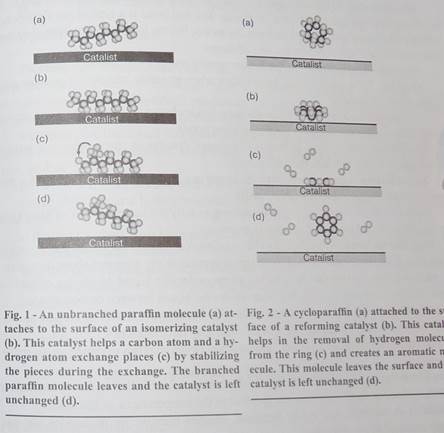
Catalysts also help individual hydrocarbon molecules to change their structures, processes called isomerization and reforming. Long unbranched paraffin molecules and cycloparaffins aren't useful in gasoline because they cause knocking. Isomeriz-ing catalysts, usually platinum, help the unbranched molecules to rearrange into highly branched molecules (Fig. 1). Reforming catalysts, usually platinum and rhenium, assist in converting cycloparaffins into aromatics. In both cases, the octane numbers increase substantially. Much of the low octane, raw gasoline obtained from the first distillation tower is subsequently sent through catalytic isomerizing and reforming facilities to increase its octane number.
The goal of isomerization is to add more branches to a paraffin molecule by interchanging hydrogen atoms and carbon atoms. On the isomerizing catalyst's surface, one carbon atom and one hydrogen temporarily let go of the hydrocarbon molecule and exchange places. Several such interchanges turn the molecule into highly branched paraffin with a high octane number.
Without a catalyst, this isomerizing process requires a great deal of energy. Two separate covalent bonds must break completely so that the pieces become free radicals. The carbon and hydrogen atoms must then exchange places and reattach to the main portion of the molecule. This complicated process is unlikely to happen, even at high temperatures.
The isomerizing catalyst facilitates the process by binding temporarily to the molecule and its fragments. The various pieces never become free radicals. Instead, they migrate along the surface of the catalyst and eventually reattach to one another without ever being completely free. The catalyst even helps the fragments stay close enough together to exchange places. What would otherwise be an almost impossible event becomes rather likely.
A reforming catalyst helps cycloparaffin molecules get rid of hydrogen atoms and become aromatics (Fig, 2), Aromatics have higher octane numbers than cycloparaffins, so this reforming is important for gasoline. Although catalysts ease the removal of the hydrogen atoms as hydrogen molecules, the final product molecules have more chemical potential energy than the original molecules. Because this reaction converts a significant amount of thermal energy into chemical potential energy, heat must be added to keep it going.
In addition to isomerization and reforming, oil refineries also use Cfttalyststo attach smaller molecules together to form larger molecules. Catalytic akylation and polymerization are used to form gasoline molecules from smaller molecules that would otherwise be difficult to use. Both processes start with olefin molecules produced in thermal or catalytic cracking. The olefin molecules have reactive double bonds, and catalysts encourage them to stick to one another or to other molecules. These reactions produce highly branched, high octane gasoline.
Дата добавления: 2017-10-04; просмотров: 1415;











