INTRODUCTION TO NUCLEAR POWER
To provide the power for a dynamo-electric machine, or electric generator, nuclear power plants rely on the process of nuclear fission. In this process, the nucleus of a heavy element, such as uranium, splits when bombarded by a free neutron in a nuclear reactor. (1) The fission process for uranium atoms yields two smaller atoms, one to three free neutrons, plus an amount of energy. Because more free neutrons are released from a uranium fission event than are required to initiate the event, the reaction can become self sustaining - a chain reaction-under controlled conditions, thus producing a tremendous amount of energy.
In the vast majority of the world's nuclear power plants, heat energy generated by burning uranium fuel is collected in ordinary water and is carried away from the reactor's core either as steam in boiling water reactors or as superheated water in pressurized-water reactors. In a pressurized-water reactor, the superheated water in the primary cooling loop is used to transfer heat energy to a secondary loop for the creation of steam. In either a boiling-water or pressurized-water installation, steam under high pressure is the medium used to transfer the nuclear reactor's heat energy to a turbine that mechanically turns a dynamo- electric machine, or electric generator. Boiling-water and pressurized-water reactors are called light-water reactors, because they utilize ordinary water to transfer the heat energy from reactor to turbine in the electricity generation process. In other reactor designs, the heat energy is transferred by pressurized heavy water, gas, or another cooling substance.
Because the water used to remove heat from the core m a light-water reactor absorbs some of the free neutrons normally generated during operation of the reactor, the concentration of the naturally fissionable 235U isotope in uranium used to fuel light-water reactors must be increased above the level of natural uranium to assist in sustaining the nuclear chain reaction in the reactor core; the remainder of the uranium in the fuel is 238U. Increasing the concentration of 235U in nuclear fuel uranium above the level that occurs in natural uranium is accomplished through the process of enrichment which is explained below.
The fuel core for a light-water nuclear power reactor can have up to 3,000 fuel assemblies. An assembly consists of a group of sealed fuel rods, each filled with U02 pellets, held in place by end plates and supported by metal spacer-grids to brace the rods and maintain the proper distances between them. The fuel core can be thought of as a reservoir from which heat energy can be extracted through the nuclear chain reaction process. During the operation of the reactor, the concentration of235U in the fuel is decreased as those atoms undergo nuclear fission to create heat energy. Some 23 8U atoms are converted to atoms of fissile 239Pu, some of which will, in turn, undergo fission and produce energy. The products created by the nuclear fission reactions are retained within the fuel pellets and these become neutron-absorbing products (called "poisons") that act to slow the rate of nuclear fission and heat production. As the reactor operation is continued, a point is reached at which the declining concentration of fissile nuclei in the fuel and the increasing concentration of poisons result in lower than optimal heat energy generation, and the reactor must be shut down temporarily and refueled.
The amount of energy in the reservoir of nuclear fuel is frequently expressed in terms of "full-power days," which is the number of 24-hour periods (days) a reactor is scheduled, for operation at full power output for the generation of heat energy. The number of full power days in a reactor's operating cycle (between refueling outage times) is related to the amount of fissile 23 5U contained in the fuel assemblies at the beginning of the cycle. A higher percentage of235U in the core at the beginning of a cycle will permit the reactor to be run for a greater number of full power days.
At the end of the operating cycle, the fuel in some of the assemblies is "spent," and it is discharged and replaced with new (fresh) fuel assemblies. The fraction of the reactor's fuel core replaced during refueling is typically one-fourth for a boiling-water reactor and one-third for a pressurized-water reactor.
The amount of energy extracted from nuclear fuel is called its "bum up," which is expressed in terms of the heat energy produced per initial unit of fuel weight. Bum up is commonly expressed as megawatt days thermal per metric ton of initial heavy metal.
The Nuclear Fuel Cycle,The nuclear fuel cycle for typical light-water reactors is illustrated in Figure AJ. The cycle consists of "front end" steps that lead to the preparation of uranium for use as fuel for reactor operation and "back end" steps that are necessary to safely manage, prepare, and dispose of the highly radioactive spent nuclear fuel. Chemical processing of the spent fuel material to recover the remaining fractions of fissionable products, 235U and 239Pu, for use in fresh fuel assemblies is technically feasible. Reprocessing of spent commercial-reactor nuclear fuel is not permitted in the United States. The front end of the nuclear fuel cycle commonly is separated into the following steps.
Exploration. A deposit of uranium, discovered by geophysical techniques, is evaluated and sampled to determine the amounts of uranium materials that are extractable at specified costs from the deposit Uranium reserves are the amounts of до that we estimated to be recoverable at stated costs.
Mining. Uranium ore can be extracted through conventional mining in open pit and underground methods similar to those used for mining other metals. In situ leach mining methods also are used to mine uranium in the United States. In this technology, uranium is leached from the in-place ore through an array of regularly spaced wells and is then recovered from the leach solution at a surface plant. Uranium ores in the United States typically range from about 0.05 to 0.3 percent uranium oxide (U30g). Some uranium deposits developed in other countries are of higher grade and are also larger than deposits mined in the United States. Uranium is also present in very low grade amounts (50 to 200 parts per million) in some domestic phosphate-bearing deposits of marine origin. Because very large quantities of phosphate-bearing rock are mined for the production of wet-process phosphoric acid used in high analysis fertilizers and other phosphate chemicals, at some phosphate processing plants the uranium, although present in very low concentrations, can be economically recovered from the process stream.
Milling. Mined uranium ores normally are processed by grinding the ore materials to a uniform particle size and then treating the ore to extract the uranium by chemical leaching. The milling process commonly yields dry powder-form material consisting of natural uranium, "yellowcake," which is sold on the uranium market as U3 Оg
Uranium conversion.Milled uranium oxide, U30g , must be converted to uranium hexafluoride, UF6, which is the form required by most commercial uranium enrichment facilities currently in use. A solid at room temperature, UF6 can be changed to a gaseous form at moderately higher temperatures. The UF6 conversion product contains only natural, not enriched, uranium.
Enrichment. The concentration of the fissionable isotope, 235U (0.71 percent in natural uranium) is less than that required to sustain a nuclear chain reaction in light water reactor cores. Natural UF6 thus must be "enriched" in the fissionable isotope for it to be used as nuclear fuel. The different levels of enrichment required for a particular nuclear fuel application are specified by the customer: light-water reactor fuel normally is enriched up to about 4 percent 235U, but uranium enriched to lower concentrations also is required. Gaseous diffusion and gas centrifuge are the commonly used uranium enrichment technologies. The gaseous diffusion process consists of passing the natural UF6 gas feed under high pressure through a series of diffusion barriers (semi porous membranes) that permit passage of the lighter 235UF6 atoms at a faster rate than the heavier 238UF6 atoms.
This differential treatment, applied across a large number of diffusion "stages," progressively raises the product stream concentration of 235U relative to 238U. lathe gaseous diffusion technology, the separation achieved per diffusion stage is relatively low, and a large number of stages is required to achieve the desired level of isotope enrichment. Because this technology requires a large capital outlay for facilities and it consumes large amounts of electrical energy, it is relatively cost intensive. In the gas centrifuge process, the natural UF6 gas is spun at high speed in ft series, of cylinders. This acts to separate the 235UF6 and |238UF6 atoms based on their slightly different atomic masses. Gas centrifuge technology involves relatively high capital costs for the specialized equipment required, but its power costs are below those for the gaseous diffusion technology. New enrichment technologies currently being developed are the atomic vapor laser isotope separation (AVLIS) and the molecular laser isotope separation (MLIS). Each laser-based enrichment process can achieve higher initial enrichment (isotope separation) factors than the diffusion or centrifuge processes can achieve. Both AVLIS and MLIS will be capable of operating at high material throughput rates.
Fabrication. For use as nuclear fuel, enriched UF6 is converted into uranium dioxide (U02) powder which is then processed into pellet form. The pellets are then fired in a high temperature sintering furnace to create hard, ceramic pellets of enriched uranium. The cylindrical pellets then undergo a grinding process to achieve a uniform pellet size. The pellets are stacked, according to each nuclear core's design specifications, into tubes of corrosion-resistant metal alloy. The tubes are sealed to contain the fuel pellets: these tubes are called fuel rods. The finished fuel rods are grouped in special fuel assemblies that are then used to build up the nuclear fuel core of a power reactor.
The back end of the cycle is divided into the following steps:
Interim Storage. After its operating cycle, the reactor is shut down for refueling. The fuel discharged at that time (spent fuel) is stored either at the reactor site or, potentially, in a common facility away from reactor sites. If on-site pool storage capacity is exceeded, it may be desirable to store aged fuel in modular dry storage facilities known as Independent Spent Fuel Storage Installations (ISFSI) at the reactor site or at a facility away from the site. The spent fuel rods are usually stored in water, which provides both cooling (the spent fuel continues to generate heat as a result of residual radioactive decay) and shielding (to protect the environment from residual ionizing radiation).
Reprocessing. Spent fuel discharged from light-water reactors contains appreciable 'quantities of fissile (U-235, Pu-239), fertile (U-238), and other radioactive materials. These fissile and fertile materials can be chemically separated and recovered from the spent fuel. The recovered uranium and plutonium can, if economic and institutional conditions permit, be recycled for use as nuclear fuel. Currently, plants in Europe are reprocessing spent fuel from utilities in Europe and Japan.
Waste Disposal. A current concern in the nuclear power field is the safe disposal and isolation of either spent fuel from reactors or, if the reprocessing option is used, wastes from reprocessing plants. These materials must be isolated from the biosphere until the radioactivity contained in them has diminished to a safe level. Under the Nuclear Waste Policy Act of 1982, as amended, the Department of Energy has responsibility for the development of the waste disposal system for spent nuclear fuel and high-level radioactive waste. Current plans call for the ultimate disposal of the wastes in solid form in licensed deep, stable geologic structures.
Uranium in nature consists primarily of two isotopes, 23 8U and 23 5U. The numbers refer to the atomic mass for each isotope, or the number of protons and neutrons in the atomic nucleus. Naturally occurring uranium consists of approximately 99.28 percent 238U and 0.71 percent 235U. The atomic nucleuses of 235U will nearly always fission when struck by a free neutron, and the isotope is therefore said to be a "fissile" isotope is therefore said to be a <fissile> isotope.
The nucleus of a 238U atom on the other hand, rather than undergoing fission when struck by a free neutron, will nearly always absorb the neutron and yield an atom of the isotope 239U. This isotope then undergoes natural radioactive decay to yield 239Pu, which, like 235U, is a fissile isotope. The atoms of 238U are said to be fertile, because, through neutron irradiation in the core, some eventually yield atoms of fissile 239Pu.
WHAT IS URANIUM?
• Uranium is a very heavy (dense) metal which can be used as an abundant source of concentrated energy.
• It occurs in most rocks in concentrations of 2 to 4 parts per million and is as common in the earth's crust as tin, tungsten and molybdenum. It occurs in seawater, and could be recovered from the oceans if prices rose significantly.
• It was discovered in 1789 by Martin Klaproth, a German chemist, in the mineral called pitchblende. It was named after the planet Uranus, which had been discovered eight years earlier.
• Uranium was apparently formed in super novae about 6.6 billion years ago. While it is not common in the solar system, today its radioactive decay provides the main source of heat inside the earth, causing convection and continental drift
• The high density of uranium means that it also finds uses in the keels of yachts and as counterweights for aircraft control surfaces (rudders and elevators), as well as for radiation shielding.
• Its melting point is 1132°C. The chemical symbol for uranium is U.
The Uranium Atom. On a scale arranged according to the increasing mass of their nuclei, uranium is the heaviest of all the naturally-occurring elements (Hydrogen is the lightest). Uranium is 18.7 times as dense as water. Like other elements, uranium occurs in slightly differing forms known as "isotopes'. These isotopes (16 in the case of uranium) differ from each other in the number of particles (neutrons) in the nucleus. 'Natural' uranium as found in the earth's crust is a mixture largely of two isotopes: urauum-238 (U-238), accounting for 99.3% and U-235 about 0.7%.
The isotope U-235 is important because under certain conditions it can readily be split, yielding a lot of energy. It is therefore said to be 'fissile* and we use the expression 'nuclear fission'
Meanwhile, like all radioactive isotopes, it decays. U-238 decays very slowly, its half-life being the same as the age of the earth (4500 million years). This means that it is barely radioactive, less so than many other isotopes in rocks and sand. Nevertheless it generates 0.1 watts/tonne and this is enough to warm the earth's core.
Energy from the uranium atom.The nucleus of the U-23S atom comprises 92 protons and 143 neutrons (92 + 143 ж 235). When the nucleus of a U-235 atom captures a neutron it splits in two (fissions) and releases some energy in the form of heat, also two or three additional neutrons are thrown off. If enough of these expelled neutrons cause the nuclei of other U-235 atoms to split, releasing further neutrons, fission 'chain reaction^ can be achieved. When this happens over and over again, many millions of times, a very large amount of heat is produced from a relatively small amount of uranium.
It is this process, in effect "burning" uranium, which occurs in a nuclear reactor. The heat is used to make steam to produce electricity.
Inside the reactor
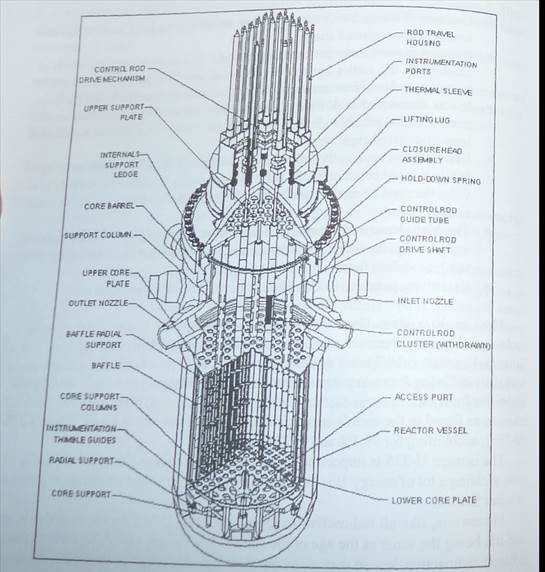
In a nuclear reactor the uranium fuel is assembled in such a way that a controlled fission chain reaction сад be achieved The heat created by splitting the U-235 atoms is then used to make steam which spins a turbine to drive a generator, producing electricity.
Nuclear power stations and fossil-fuelled power stations of similar capacity have many features in common. Both require heat to produce steam to drive turbines and generators. In a nuclear power station, however, the fisioning of uranium atoms replaces the burning of coal or gas. The chain reaction that takes place in the core of a nuclear reactor is controlled by rods which absorb neutrons and which can be inserted or withdrawn to set the reactor at the required power level. The fuel elements are surrounded by a substance called a moderator to slow the speed of the emitted neutrons and thus enable the chain reaction to continue. Water, graphite and heavy water are used as moderators in different types of reactors. Because of the kind of fuel used (i.e. the concentration of U-235, see below), if there is a major uncorrected malfunction in a reactor the fuel may melt, but it cannot explode like a bomb. A typical 1000 megawatt (MW) reactor can provide enough electricity for a modern city of close to one million people. About 35 such nuclear reactors could provide Australia's total electricity needs.
Дата добавления: 2017-10-04; просмотров: 2064;











