THERMAL AND CATALYTIC CRACKING
Unfortunately, just sorting the molecules in crude oil isn't good enough for most refineries. The principal outputs of these refineries are transportation fuels and there is comparatively little market for the other molecules in crude oil. Since less than half of the molecules of crude oil are suitable for transportation fuels, the refinery has a problem. Moreover, it can't store the unmarketable molecules indefinitely. While the refinery burns some of the less useful molecules to provide its own power, it must sell everything else to make room for incoming crude oil. So large integrated refineries have facilities for converting the less useful molecules in crude oil into ones it can sell.
The original method for converting larger molecules into smaller molecules is thermal cracking. Above about 360°C, hydrocarbon molecules decompose into fragments. At that temperature, the random thermal energy in a hydrocarbon molecule is occasionally large enough to break that molecule into two pieces. After a short time as a free radical, each fragment rearranges into something that's chemically stable. Most of that time the new molecules are smaller than the old molecules.
The higher the temperature, the more often such decompositions occur and the faster the petroleum cracks. While thermal cracking is a nuisance to be avoided in distillation, it's valuable when done in a controlled manner in a cracking tank. The big molecules that aren't suitable for gasoline generally decompose into smaller ones that are.
Moreover, thermal cracking produces many olefin molecules that have higher octane numbers than the usual contents of crude oil. These olefin molecules are made when the free radical fragments of original hydrocarbon molecules rearrange internally to form double bonds. If the last carbon atom in a chain has only three neighbors, it can complete its electronic shell by forming a double bond with the carbon atom next to it. This rearrangement causes the neighboring carbon to abandon a hydrogen atom, which immediately becomes part of a hydrogen molecule. So thermal cracking creates many smaller molecules, with double bonds at their ends, and hydrogen molecules.
But thermal cracking is difficult to control and also creates many large and useless molecules. As a rule, the higher the temperature in the cracking tank, the higher the octane of the gasoline it produces but the smaller the yield. To make premium gasoline by thermal cracking, the refinery might have to waste all but 20% of the hydrocarbons it feeds to the cracking tank. Because this waste is intolerable, thermal cracking has been replaced almost completely by fluid catalytic cracking and reforming.
In these processes, hot hydrocarbon molecules are brought alumina catalysts. Like all catalysts, these materials facilitate reducing the activation energies needed to complete them. When a hydrocarbon molecule attaches to the surface of the catalyst, the catalyst helps it rearrange (Fig. 1). The catalyst reduces the potential energies of the partially rearranged molecules so that less overall energy is needed to complete the rearrangement. Catalyzed rearrangements are proceeded at lower temperatures.
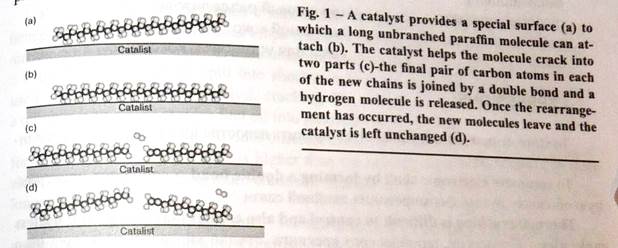
These catalysts also help to control the rearrangements. A particular catalyst will assist certain rearrangements more than others. Catalysts are particularly helpful in cracking larger molecules into smaller ones so that yields of gasoline molecules are much higher with catalysts than without.
Because all of the catalyst's work is done by its surface, most commercial catalysts are designed to have lots of surface area. The silica-alumina catalysts used in fluid catalytic cracking are actually small particles of porous materials. These particles are only about 50 microns in diameter and they swirl around with the fluid they are cracking.
The reactions take only a few seconds to complete, after which the catalyst particles must be separated from the fluid. The mixture passes through a cyclone separator, where it moves very rapidly around in a circle. The acceleration causes the denser catalyst particles to migrate to the outside of the separator and the clear fluid can then be extracted from the middle of the device.
Unfortunately, the catalyst particles quickly accumulate a coating of very targe molecules that don't react and can't be removed easily. Like most catalysts, they lose their catalytic activity when their surfaces become dirty. The only effective way to clean the surfaces of these particles is to burn the residue off them. That's just what the oil refinery does. This burning regenerates the catalyst particles and prepares them for their next trip through the fluid.
Дата добавления: 2017-10-04; просмотров: 1419;











