THERMAL FISSION REACTORS
The basic concept of a nuclear reactor is simple: assemble a critical mass of fissionable material and adjust its criticality to maintain a steady fission rate. But what should the fissionable material be? In a fission bomb, it must be relatively pure 235 U or 239 Pu. But in a fission reactor, it can be a mixture of 235 U and 238 U. It can even be natural uranium. The trick is to use thermal neutrons - slow moving neutrons that have only the kinetic energy associated with the local temperature.
In a fission bomb, 238 U is a serious problem because it captures the fast moving neutrons emitted by fissioning U235 nuclei. Natural uranium can't sustain a chain reaction because its many U238 nuclei gobble up most of the fast moving neutrons before they can induce fissions in the rare 235 U nuclei. The uranium must be enriched, so that it contains more than the natural abundance of 235 U.
But slow moving neutrons have a different experience as they travel through natural uranium. For complicated reasons, the 235 U nuclei seek out slow moving neutrons and capture them with unusual efficiency. 235 U nuclei are so good at catching slow moving neutrons that they easily win out over the more abundant 238U nuclei. Even in ^ natural uranium, a slow moving neutron is more likely to be caught by a 235 U nucleus A than it is by a 238 U nucleus. As a result, it's possible to sustain a nuclear fission chain reaction in natural uranium if all of the neutrons are slow moving.
But the previous paragraph seems to be hypothetical because 235 U nuclei emit fast moving neutrons when they fission. That's why pure natural uranium can't be used in a fission bomb. However, most nuclear reactors don't use pure natural uranium. They use natural uranium plus another material that's called a moderator. The moderator's job is to slow the neutrons down so that 235 U nuclei can grab them. A fast moving neutron from a fissioning 235 U nucleus enters the moderator, rattles around for about a thousandth of a second, and emerges as a slow moving neutron, one with only thermal energy left. It then induces fission in another 235 U nucleus. Once the moderator is present, even natural uranium can sustain a chain reaction! Reactors that carry out their chain reactions with slow moving or thermal neutrons are called thermal fission reactors.
To be a good moderator, a material must simply remove energy and momentum from the neutrons without absorbing them. When a fission neutron leaves a good moderator, it has only thermal energy left. The best moderators are nuclei that rarely or never absorb neutrons and don't fall apart during collisions with them. Hydrogen (1H), deuterium (2H), helium (4He), and carbon (12C) are all good moderators. When a fast moving neutron hits the nucleus of one of these atoms, the collision resembles that between two billiard balls. Because the fast moving neutron transfers some of its energy and momentum to the nucleus, the neutron slows down while the nucleus speeds up.
Water, heavy water (water containing the heavy isotope of hydrogen: deuterium or 2H), and graphite (carbon) are the best moderators for nuclear reactors. They slow neutrons down to thermal speeds without absorbing many of them. Of these moderators, heavy water is the best because it slows the neutrons quickly yet doesn't absorb them at all. However, heavy water is expensive because only 0.015% of hydrogen atoms are deuterium and separating that deuterium from ordinary hydrogen is difficult.
Graphite moderators were used in many early reactors because graphite is cheap and easy to work with. However, graphite is a less efficient moderator than heavy water, so graphite reactors had to be big. Furthermore, graphite can burn and was partly responsible for two of the world's three major reactor accidents. Normal or "light" water is cheap, safe, and an efficient moderator, but it absorbs enough neutrons that it can't be used with natural uranium. For use in a light water reactor, uranium must be enriched slightly, to about 2-3% 235 U.
The core of a typical thermal fission reactor consists of small uranium oxide (U02) fuel pellets, separated by layers of moderator (Fig. 1). A neutron released by a fissioning 235 U nucleus usually escapes from its fuel pellet, slows down in the moderator, and then induces fission in a 235 U nucleus in another fuel pellet. By absorbing some of these neutrons, the control rods determine whether the whole core is subcritical, critical, or supercritical. The 238 U nuclei are basically spectators in the reactor since most of the fissioning occurs in the 235 U nuclei.
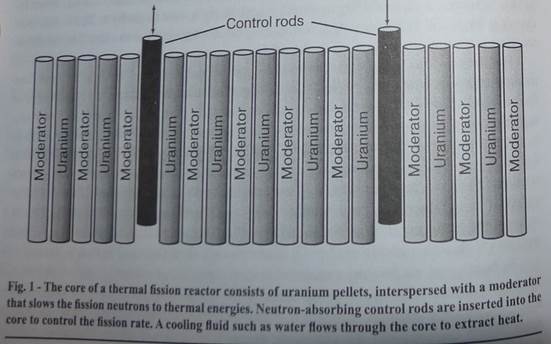
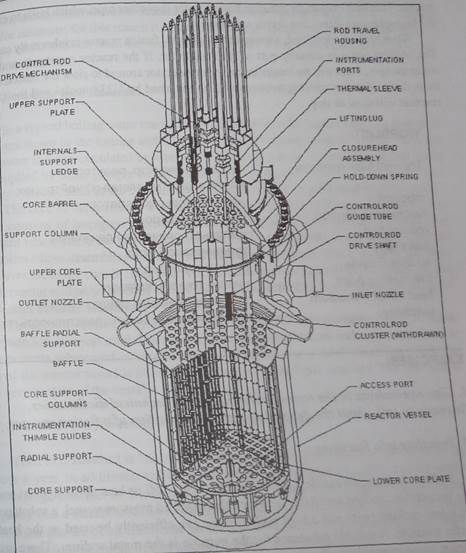
Fig.2. A cutaway drawing of the fission reactor.
In a practical thermal fission reactor, something must extract the heat released by nuclear fission. In many reactors, cooling water passes through the core at high speeds Heat flows into this water and increases its temperature. In a boiling water reactor, the water boils directly in the reactor core, creating high-pressure steam that drives the turbines of an electric generator. In a pressurized water reactor, the water is urn under enormous pressure so it can’t boil. Instead, it's pumped to a heat exchanger outside the reactor. This heat exchanger transfers heat t6 water in another pipe, which boils to create the high-pressure steam that drives a generator.
When properly designed, a water-cooled thermal fission reactor is inherently stable. The cooling water is actually part of the moderator. If the reactor overheats and the water escapes, there will no longer be enough moderator around to slow the fission neutrons down. The fast moving neutrons will be absorbed by U238 nuclei and the chain reaction will slow or stop.
Дата добавления: 2017-10-04; просмотров: 1592;











