WHAT ARE PETROLEUM PRODUCTS?
Unfortunately, crude oil isn't very useful in its raw form and must be processed extensively before it's marketable. This processing is the job of an oil refinery. But before we examine how oil refineries work, we must first consider the products they're trying to produce. Each petroleum product is an assortment of different molecules, selected and blended so that the finished mixture has the appropriate physical and chemical properties for the task it must perform. Here are some of the petroleum products made at refineries.
Let's start with gasoline for automobiles. To make gasoline, the refinery blends molecules that tend to be liquid at room temperature but gaseous at temperatures above about 200 oC. that bum easily and completely in the presence of sufficient air, and that are resistant to knocking. Knocking is premature ignition that occurs when fuel and air are compressed in an automobile engine cylinder. Work done on the gaseous mixture of fuel and air during compression raises its temperature, so the mixture is in danger of igniting spontaneously before the spark plug fires. A properly formulated gasoline avoids this spontaneous ignition.
While gasoline should remain a liquid at room temperature to stay in the tank, it must become a gas in a hot engine to burn efficiently. Not every hydrocarbon molecule can meet these two requirements. Some hydrocarbon molecules are more volatile than others-coflverting easily into a gas. A hydrocarbon molecule's volatility is determined mostly by its size. Small hydrocarbon molecules evaporate more easily than large hydrocarbon molecules.
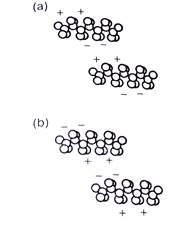
The size-dependence of volatility is related to the force holding the hydrocarbon molecules together as a liquid: the van der Waals force. This force is the result of tiny electric charge fluctuations that are present in all molecules. As electrons in two nearby molecules move about, they tend to arrange themselves so that the molecules attract one another (Fig. 1). At any given moment, the two molecules have small electrical dipoles that pull them toward one another. These dipoles come and go but they're still able to hold the molecules together.
The van der Waals forces between two molecules depend on their sizes and shapes. The larger the molecules are, the more electrons they contain and the more electrically polarizable they are. Large molecules experience stronger van der Waals forces than small molecules, which is why most small molecules are gases at room temperature while most large molecules are liquids or solids.
With gasoline, the van der Waals forces must be strong enough to keep it mostly liquid at room temperature, but weak enough to allow it to become mostly gaseous at

about 200 C. These requirements limit the sizes and shapes of the hydrocarbon molecules that gasoline can contain. The size of a hydrocarbon molecule is determined mostly by the number of carbon atoms it contains. For gasoline, the appropriate hydrocarbon molecules range from about 4 carbon atoms on the small end to about 12 carbon atoms on the large end.
Refineries adjust the precise balance of large and small molecules to give the gasoline just the right volatility over the normal range of operating temperatures. The large molecules bind together rather strongly and help to keep the gasoline liquid during storage. The small molecules are easily separated into a gas and quickly evaporate from an open container of gasoline. Butane molecules, which have only 4 caibon atoms, are included in the gasoline to make starting easy, even in a cold engine. This volatile chemical evaporates readily and is soon lost from stored gasoline. A car or lawnmower with an old tank of gas may not start because its gasoline has no more butane in it.
Because gasoline's ideal volatility depends on the outdoor temperature, the oil refineries adjust their blends according to season and climate. In winter, they reduce the average molecule size so that the gasoline vaporizes more easily in cold weather. In summer, they increase the average molecule size so that the gasoline is less prone to unwanted boiling.
But volatility isn' the only criterion for me molecules in gasoline. The other critical issue for gasoline is its resistance to knocking. Unfortunately the unbranced paraffin molecules that are common in crude oil ignite much too easily to be the major components of gasoline. Instead, most gasoline molecules are highly branched paraffins olefins, or aromatics that are difficult to ignite.
Resistance to knocking is normally characterized by a gasoline’s octane number. The higher the octane number, the harder it is to make the gasoline knock. 2,2,4-Trimethylpentane (also called "isooctane" or simply "octane")-a highly branched paraffin molecule with 8 carbon atoms is particularly resistant to knocking and is the standard by which all other molecules are measured. Its octane number is defined as 100. n-heptane, an unbranched paraffin molecule with 7 carbon atoms, knocks very easily and is the other standard. Its octane number is defined as 0.
These two hydrocarbons and their mixtures are used to assign octane numbers to gasolines. Each gasoline is compared to various mixtures of "octane" and n-heptane until a match is found. The percentage of "octane" in fhe matching mixture is then the octane number of the gasoline. For example, a gasoline that has the same knock resistance as a mixture of 90% "octane" and 10% n-heptane is given an octane number of 90. However, a gasoline's octane rating depends slightly on the conditions in which this comparison is made. The two standard conditions are research, corresponding to hard acceleration at low speeds, and motor, corresponding to zero acceleration at high-speeds. Any gasoline has two different octane numbers, its research octane number (R) and its motor octane number (M). These two octane numbers are averaged, (R+M)/2, to give the octane number that appears on the pump.
In formulating a gasoline, the refinery blends various hydrocarbons to achieve an overall octane number of about 87 for regular or 93 for premium. Since octane number only measures resistance to knocking, two different gasolines with identical octane numbers may contain very different assortments of hydrocarbon molecules. Often antiknock compounds are added to a gasoline to increase its octane number. These chemicals interfere with ignition. Tetraethyl lead was the antiknock compound of choice until concerns about lead pollution sent it into disuse. Modern antiknock additives ffiiclude tert-butyl alcohol and methyl tert-butyl ether.
Kerosene is less volatile than gasoline and consists of molecules with between 10 and 15 carbon atoms. Since kerosene is often used inside houses in lamps and space heaters, it must bum easily and cleanly, without soot or noxious odors. It's normally made from unbranched paraffins and cycloparaffins. Olefins and aromatics are difficult to burn and tend to form soot. Aromatics also tend to have strong odors.
Diesel fuel, jet fuel, and heating oil are very similar. They are even less volatile than kerosene and contain hydrocarbons with between 12 and 20 carbon atoms. While heating oil can contain just about any hydrocarbon, diesel fuel and jet fuel have to be prepared with a little more care.
In a diesel engine, liquid fuel is injected into a cylinder filled with very hot, high pressure air. The fuel must ignite easily and spontaneously and burn completely in a very short period of time. The same easy and rapid combustion is important in a jet engine. This requirement of easy ignition is just the opposite of that in a gasoline engine. The ideal diesel and jet fuel molecules are unbranched paraffin molecules such as n-cetane, which contains 16 carbon atoms. Diesel fuels are rated according to their cetane number; the extent to which the fuel resembles the easy to burn n-cetane and not the hard to bum hepfamethylnonane, a highly branched paraffin molecule that also has 16 carbon atoms.
Lubricating oils and waxesare even less volatile than fuel oils and contain molecules of between 20 and 50 carbon atoms. Pure hydrocarbons with molecules this large are normally solids at room temperature. However, lubricating oils contain so many different molecules that they're unable to find the orderly arrangements needed to form crystals. The molecules don't fit together well enough to form a rigid structure and remain a thick, viscous liquid.
Only the longer unbranched paraffin molecules are able to join together to form crystalline solids. These solids are called paraffin waxes. With time, paraffin waxes settle out of lubricating oils and are usually removed. At lower temperatures, shorter unbranched paraffin molecules also settle out of lubricating oil. The semi-solid material that forms in cold lubricating oil is petrolatum or petroleum jelly.
The remaining fluid is lubricating oil. Inserted between two movable surfaces, lubricating oil prevents those surfaces from experiencing sliding friction and wear as they slide across one another. The oil molecules cling to the surfaces and to one another with van der Waals forces and keep the two surfaces from touching. While outside forces may try to push the two surfaces together, pressure in the oil pushes back and keeps the two surfaces apart.
Oil's slipperiness comes from the nature of the forces between molecules. Apair of oil molecules is drawn together by van der Waals forces and by whatever pressure is present in the oil. However, if the molecules approach one another too closely, they begin to repel. This repulsion appears when the electron orbits of the two molecules begin to overlap. The Pauli exclusion principle doesn't allow identical electrons from both molecules to follow identical paths so it keeps the two molecules separated at an equilibrium distance.
But these forces depend only on the distance separating the two molecules and don't prevent the two molecules from sliding across one another. In fact, the molecules in oil do slide across one another quite easily and it is this mobility that makes oil such a good lubricant (Fig. 1). The van der Waals forces are virtually unaffected by sideways motion in the oil molecules.
However, when two surfaces are pushed together by outside forces, they create pressure in the oil. In a completely sealed environment, this rise in oil pressure wouldn't matter. But most lubricated surfaces have openings to the outside, where the pressure is lower. Since fluids always accelerate toward lower pressure, lubricating oil accelerates toward openings. The only thing preventing oil from squirting out from between two lubricated surfaces is the oil's viscosity-its difficulty flowing past itself. The more viscous the oil, the more it tends to remain between two surfaces to protect them from wear.
Using a lubricating oil with the right viscosity is important in many applications. If the oil isn't viscous enough, it will escape and will not protect the surfaces. If it's too viscous, energy will be wasted doing work against viscous forces, which turn that work into thermal energy.
An oil's viscosity depends strongly on the sizes of its molecules. The larger the molecules, the more viscous the oil. But molecular structure and temperature are also important. Some molecules, particularly cycloparaffins and aromatics, change their vis-cosities significantly as their temperatures change. Since most situations call for oils that don't change with temperature, most lubricating oils are composed primarily of branched paraffin molecules (Fig. 1).
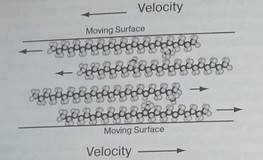 Fig. 2 - Lubricating oil molecules cling to one another with non-directional van der Waals forces and slide across one another easily. They prevent two surfaces from touching and dramatically reduce the amount of sliding friction between those surfaces.
Fig. 2 - Lubricating oil molecules cling to one another with non-directional van der Waals forces and slide across one another easily. They prevent two surfaces from touching and dramatically reduce the amount of sliding friction between those surfaces.
Motor oils frequently contain additives to maintain their viscosities at higher temperatures. These additives are long molecules that roll up into compact balls at low temperatures but open up at high temperatures. In their open forms, these molecules thicken the oil and help it do its job. At very high temperatures, these additives and the oil itself fragment into smaller molecules and permanently lose much of their viscosity. That's why it mustn't be overheated.
The largest hydrocarbon molecules that leave an oil refinery are found in asphalt. Asphalt is what's left over when all of the other hydrocarbon molecules have been separated from crude oil. Asphalt molecules may have long paraffin chains or a number of interlocking rings, and frequently include atoms other than carbon and hydrogen. This crazy mixture of giant molecules is used mostly to pave roads. Asphalt molecules are large enough that the van der Waals forces between them prevent their motion at room temperatures. They form a stiff, structureless material that clings to surfaces and makes an excellent binder for the gravel in pavement.
The last major product of oil refineries is gases. These hydrocarbon molecules are so small that van der Waals forces can't keep them together at room temperature and atmospheric pressure, and they evaporate into gas. While many of these gaseous molecules are formed during the refining process, methane, with only 1 carbon atom), occurs naturally in underground reservoirs. Methane extracted from the ground is sent through pipelines and is sold as natural gas. It's a colorless, odorless, non-toxic gas that is significantly lighter than air. Breathing it is dangerous only because it contains no oxygen. Methane is very flammable, however, so a small amount of a sulfur-based odorant is added to it to help point out leaks.
Methane can only be liquefied by cooling it to very low temperature. This limitation makes natural gas difficult to store. However propane, with 3 carbon atoms becomes liquid under pressure. Liquefied propane gas is stored in pressurized tanks and is used for heating and cooking. Liquefied petroleum gas (LP gas) contains both propane and butane.
Pressurizing the propane gas increases its density enough to sustain a liquid phase in a tank. Individual propane molecules continue to move back and forth between the liquid phase and the gaseous phase, but there's no net change in the amounts of liquid and gas. When you remove some of the propane gas from a tank to cook your food, some of the liquid evaporates to replace the missing gas molecules. This automatic replacement of the removed gas makes propane a very convenient fuel.
Дата добавления: 2017-10-04; просмотров: 2132;











