Haptens and the Study of Antigenicity
The pioneering work of Karl Landsteiner in the 1920s and 1930s created a simple, chemically defined system for studying the binding of an individual antibody to a unique epitope haptens,small organic molecules that are antigenic but not immunogenic. Chemical coupling of a hapten to a large protein, called a carrier,yields an immunogenic hapten-carrier conjugate.Animals immunized with such a conjugate produce antibodies specific for (1) the hapten determinant, (2) unaltered epitopes on the carrier protein, and (3) new epitopes formed by combined parts of both the hapten and carrier (Figure 5). By itself, a hapten cannot function as an immunogenic epitope.But when multiple molecules of a single hapten are coupled to a carrier protein (or nonimmunogenic homopolymer), the hapten becomes accessible to the immune system and can function as an immunogen.
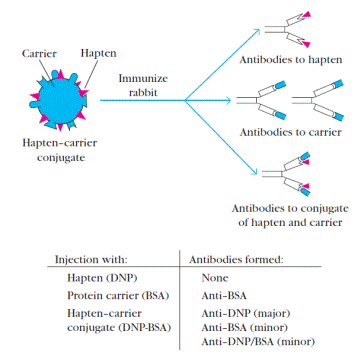
Figure 5. A hapten-carrier conjugate contains multiple copies of the hapten – a small nonimmunogenic organic compound such as dinitrophenol (DNP) – chemically linked to a large protein carrier such as bovine serum albumin (BSA). Immunization with DNP alone elicits no anti-DNP antibodies, but immunization with DNPBSA elicits three types of antibodies. Of these, anti-DNP antibody is predominant, indicating that in this case the hapten is the immunodominant epitope in a hapten-carrier conjugate, as it often is in such conjugates.
The beauty of the hapten-carrier system is that it provides immunologists with a chemically defined determinant that can be subtly modified by chemical means to determine the effect of various chemical structures on immune specificity. In his studies, Landsteiner immunized rabbits with a haptencarrier conjugate and then tested the reactivity of the rabbit’s immune sera with that hapten and with closely related haptens coupled to a different carrier protein.He was thus able to measure, specifically, the reaction of the antihapten antibodies in the immune serum and not that of antibodies to the original carrier epitopes. Landsteiner tested whether an antihapten antibody could bind to other haptens having a slightly different chemical structure. If a reaction occurred, it was called a cross-reaction.By observing which hapten modifications prevented or permitted cross-reactions, Landsteiner was able to gain insight into the specificity of antigenantibody interactions.
Using various derivatives of aminobenzene as haptens, Landsteiner found that the overall configuration of a hapten plays a major role in determining whether it can react with a given antibody. For example, antiserum from rabbits immunized with aminobenzene or one of its carboxyl derivatives (o-aminobenzoic acid, m-aminobenzoic acid, or paminobenzoic acid) coupled to a carrier protein reacted only with the original immunizing hapten and did not cross-react with any of the other haptens. In contrast, if the overall configuration of the hapten was kept the same and the hapten was modified in the para position with various nonionic derivatives, then the antisera showed various degrees of cross-reactivity. Landsteiner’s work not only demonstrated the specificity of the immune system, but also demonstrated the enormous diversity of epitopes that the immune system is capable of recognizing.
Many biologically important substances, including drugs, peptide hormones, and steroid hormones, can function as haptens. Conjugates of these haptens with large protein carriers can be used to produce hapten-specific antibodies. These antibodies are useful for measuring the presence of various substances in the body. For instance, the original home pregnancy test kit employed antihapten antibodies to determine whether a woman’s urine contained human chorionic gonadotropin (HCG), which is a sign of pregnancy. However, as shown in the Clinical Focus, the formation of drug-protein conjugates in the body can produce drug allergies that may be life-threatening.
Дата добавления: 2016-07-18; просмотров: 2853;











