Explore the development of the fuel cell and study the different systems.
BU-210: How does the Fuel Cell Work?
A fuel cell is an electrochemical device that combines hydrogen fuel with oxygen to produce electricity, heat and water. The fuel cell is similar to a battery in that an electrochemical reaction occurs as long as fuel is available. Hydrogen is stored in a pressurized container and oxygen is taken from the air. Because of the absence of combustion, there are no harmful emissions, and the only by-product is pure water. So pure is the water emitted from the proton exchange membrane fuel cell (PEMFC) that visitors to Vancouver’s Ballard Power Systems were served hot tea made from this clean water.
Fundamentally, a fuel cell is electrolysis in reverse, using two electrodes separated by an electrolyte. The anode (negative electrode) receives hydrogen and the cathode (positive electrode) collects oxygen. A catalyst at the anode separates hydrogen into positively charged hydrogen ions and electrons. The oxygen is ionized and migrates across the electrolyte to the anodic compartment, where it combines with hydrogen. A single fuel cell produces 0.6–0.8V under load. To obtain higher voltages, several cells are connected in series. Figure 1 illustrates the concept of a fuel cell.
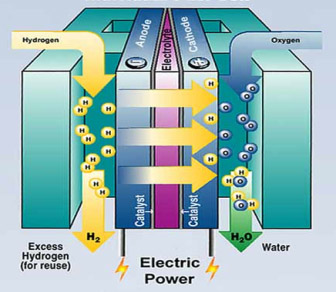
| Figure 1: Concept of a fuel cell. The anode (negative electrode) receives the hydrogen and the cathode (positive electrode) collects the oxygen. Source: US Department of Energy, office of Energy Efficiency and Renewable Energy |
Fuel cell technology is twice as efficient as combustion in turning carbon fuel to energy. Hydrogen, the simplest chemical element (one proton and one electron), is plentiful and exceptionally clean as a fuel. Hydrogen makes up 90 percent of the universe and is the third most abundant element on the earth’s surface. Such a wealth of fuel would provide an almost unlimited pool of clean energy at relatively low cost. But there is a hitch.
With most fuels, hydrogen is bonded to other substances and “unleashing” the gas takes energy. In terms of net calorific value (NCV), hydrogen is more costly to produce than gasoline. Some say that hydrogen is nearly energy neutral, meaning that it takes as much energy to produce as it delivers at the end destination. (See BU-1007: Net Calorific Value.)
Storage of hydrogen poses a further disadvantage. Pressurized hydrogen requires heavy steel tanks, and the NCV by volume is about 24 times lower than a liquid petroleum product. In liquid form, which is much denser, hydrogen needs extensive insulation for cold storage.
Hydrogen can also be produced with a reformer by means of extraction from an existing fuel, such as methanol, propane, butane or natural gas. Converting fossil fuel into pure hydrogen releases some leftover carbon, but this is 90 percent less harmful than what comes from the tailpipe of a car. Carrying a reformer would add weight to the vehicle and increase its cost; reformers are also sluggish. The net benefit of hydrogen conversion is in question because it does not solve the energy problem.
With the availability of hydrogen through extraction, the fuel cell core (stack) to convert hydrogen and oxygen to electricity is expensive and the stack has a limited life span. Burning fossil fuels in a combustion engine is the simplest and most effective means of harnessing energy, but this contributes to pollution.
Sir William Grove, a Welsh judge and gentleman scientist, developed the fuel cell concept in 1839, but the invention never took off. This was during the development of the internal combustion engine (ICE) that showed promising results. It was not until the 1960s that the fuel cell was put to practical use during the Gemini space program. NASA preferred this clean power source to nuclear or solar power. The alkaline fuel cell system that was chosen generated electricity and produced drinking water for the astronauts.
High material costs made the fuel cell prohibitive for commercial use. The fuel cell core (stack) is expensive and has a limited life span. Burning fossil fuel in a combustion engine is the simplest and most effective means to harness energy, but it pollutes.
High cost did not discourage the late Karl Kordesch, the co-inventor of the alkaline battery, from converting his car to an alkaline fuel cell in the early 1970s. He mounted the hydrogen tank on the roof and placed the fuel cell and backup batteries in the trunk. According to Kordesch, there was enough room for four people and a dog. He drove his car for many years in Ohio, USA, but the only problem, Kordesch told me in person, was that the car did not pass inspections because it had not tail pipe.
Here are the most common fuel cell concepts.
Дата добавления: 2016-12-16; просмотров: 1791;











