Main Periods of Immunology Development
1. The roots of immunology
The discipline of immunology grew out of the observation that individuals who had recovered from certain infectious diseases were thereafter protected from the disease. The Latin terms immunis, meaning “exempt, released” and immunitas – liberation, freedom from something are the source of English word immunity, meaning the state of protection from infectious disease or invasive alien species (including pathogens), as well as foreign substances having antigenic properties.
Immunity is the way of organism protection against substances with foreign genetic material, with foreign or own, but changed, antigens. Immunology is the science of immunity. It deals with genetic, molecular and cellular mechanisms of organism reaction on foreign substances, or antigens.
Perhaps the earliest written reference to the phenomenon of immunity can be traced back to Thucydides (Фукидид), the great historian of the Peloponnesian War (Пелопоннесская война). In describing a plague (чума) in Athens, he wrote in 430 BC that only those who had recovered from the plague could nurse the sick because they would not contract the disease a second time. Although early societies recognized the phenomenon of immunity, almost two thousand years passed before the concept was successfully converted into medically effective practice.
The first recorded attempts to induce immunity deliberately (сознательно) were performed by the Chinese and Turks in the fifteenth century. Various reports suggest that the dried crusts (корки) derived from smallpox pustules were either inhaled into the nostrils or inserted into small cuts in the skin (a technique called variolation).
In 1718, Lady Mary Wortley Montagu, the wife of the British ambassador (посол) to Constantinople, observed the positive effects of variolation on the native population and had the technique performed on her own children.
The method was significantly improved by the English physician Edward Jenner, in 1798. Intrigued by the fact that milkmaids who had contracted the mild disease cowpox were subsequently immune to smallpox, which is a disfiguring and often fatal disease, Jenner reasoned that introducing fluid from a cowpox pustule into people (i.e., inoculating them) might protect them from smallpox. To test this idea, he inoculated an eight-year-old boy with fluid from a cowpox pustule and later intentionally infected the child with smallpox. As predicted, the child did not develop smallpox. Jenner’s technique of inoculating with cowpox to protect against smallpox spread quickly throughout Europe. However, for many reasons, including a lack of obvious disease targets and knowledge of their causes, it was nearly a hundred years before this technique was applied to other diseases.
As so often happens in science, serendipity (интуитивная прозорливость) in combination with astute (проницательный) observation led to the next major advance in immunology, the induction of immunity to cholera. Louis Pasteur had succeeded in growing the bacterium thought to cause fowl (птица) cholera (т.е. куриная холера) in culture and then had shown that chickens injected with the cultured bacterium developed cholera. After returning from a summer vacation, in 1881, he injected some chickens with an old culture. The chickens became ill, but, to Pasteur’s surprise, they recovered. Pasteur then grew a fresh culture of the bacterium with the intention (намерение) of injecting it into some fresh chickens. But, as the story goes, his supply of chickens was limited, and therefore he used the previously injected chickens. Again to his surprise, the chickens were completely protected from the disease. Pasteur hypothesized and proved that aging had weakened the virulence of the pathogen and that such an attenuated strain might be administered to protect against the disease. He called this attenuated strain a vaccine(from the Latin vacca, meaning “cow”), in honor of Jenner’s work with cowpox inoculation.
Pasteur extended these findings to other diseases, demonstrating that it was possible to attenuate,or weaken a pathogen and administer the attenuated strain as a vaccine. In a now classic experiment at Pouilly-le-Fort (в Пуйи-ле-Фор) in 1881, Pasteur first vaccinated one group of sheep with heat-attenuated anthrax (сибирская язва) bacillus (Bacillus anthracis); he then challenged the vaccinated sheep and some unvaccinated sheep with a virulent culture of the bacillus. All the vaccinated sheep lived, and all the unvaccinated animals died. These experiments marked the beginnings of the discipline of immunology. In 1885, Pasteur administered his first vaccine to a human, a young boy who had been bitten repeatedly by a rabid (бешеный) dog. The boy, Joseph Meister, was inoculated with a series of attenuated rabies virus preparations. He lived and later became a custodian (сторож) at the Pasteur Institute.
2. Early studies revealed (showed, demonstrated)humoral and cellular components of the immune system
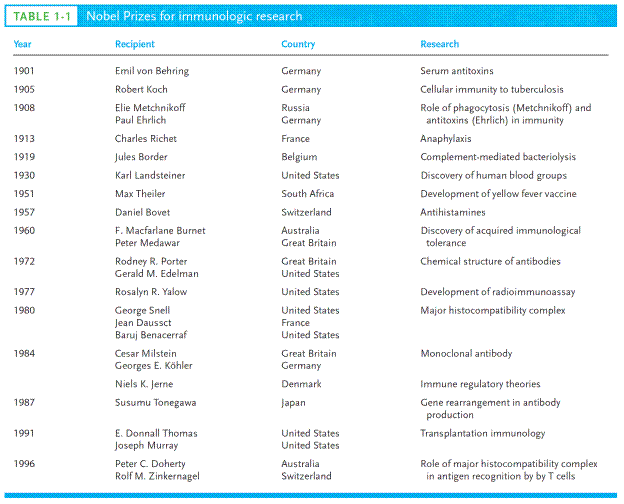
Although Pasteur proved that vaccination worked, he did not understand how. The experimental work of Emil von Behring (Эмиль фон Беринг) and Shibasaburo Kitasato (Сибасабуро Китасато) in 1890 gave the first insights (ideas) into the mechanism of immunity, earning von Behring the Nobel prize in medicine in 1901 (Table 1-1). Von Behring and Kitasato demonstrated that serum(the liquid, noncellular component of coagulated blood) from animals previously immunized to diphtheria could transfer the immune state to unimmunized animals. In search of the protective agent, various researchers during the next decade demonstrated that an active component from immune serum could neutralize toxins, precipitate toxins, and agglutinate (clump – глыба) bacteria. In each case, the active agent was named for the activity it exhibited: antitoxin, precipitin, and agglutinin, respectively.
| Год присуждения премии | Лауреат | За что присуждена премия |
| Эмиль Адольф фон Беринг | За открытие антитоксинов (антител), их применение при лечении дифтерии. | |
| Роберт Кох | За исследование туберкулеза. | |
| Илья Ильич Мечников и Пауль Эрлих | За труды по иммунитету, открытие фагоцитоза (Мечников) и гуморальную теорию иммунитета (Эрлих). | |
| Шарль Рише | В знак признания его работ по анафилаксии. | |
| Жюль Борде | За экспериментальные работы по комплементзависимому бактериолизу, специфическому гемолизу, за разработку метода фиксации комплемента для диагностики инфекционных болезней. | |
| Карл Ландштейнер | За открытие групп крови человека. | |
| Макс Тейлер | За создание вакцины против желтой лихорадки. | |
| Даниеле Бове | За открытие роли гистамина в патогенезе аллергических реакций и разработку антигистаминных фармакологических препаратов для лечения аллергических болезней. | |
| Макфарлейн Бёрнет иПитер Брайан Медавар | За открытие искусственной иммунной толерантности (переносимости). | |
| Джералд Эдельман и Родни Портер | За открытия, касающиеся химической структуры антител. | |
| Розалин Сасмен Ялоу | За развитие радиоиммунологических методов определения пептидных гормонов. | |
| Барух Бенасерраф,Жан Доссе и Джордж Снелл | За открытие генов и структур поверхности клеток главного комплекса гистосовместимости. | |
| Нильс Ерне, Георг Кёлер и Сезар Мильштейн | За открытие и разработку принципов выработки моноклональных антител с помощью гибридов. | |
| Судзуми Тонегава | За открытие генетического принципа для генерации разновидности антител. | |
| E.Donnall Thomas, Joseph Murray | Transplantation immunology | |
| Питер Доэрти иРольф Цинкернагель | За открытия в области иммунной системы человека, в частности её способности выявлять клетки, пораженные вирусом Role of major histocompatibility complex in antigen recognition by T-cells, ability of immune system to detect virus-infected cells | |
| Стенли Прузинер Stanley B. Prusiner | За открытие прионов, нового биологического принципа инфекции For the discovery of prions, a new biological principle of infection | |
| Ральф Стейнман Ralph Steinman | За открытие дендритных клеток и изучение их значения для приобретённого иммунитета For the discovery of dendritic cells and their importance for the study of the adaptive immune system | |
| Жюль Хоффман иБрюс Бётлер Jules A. Hoffmann and Bruce Beutler | За работы по изучению активации врожденного иммунитета For studies on innate immunity activation |
In 1883, even before the discovery that a serum component could transfer immunity, Elie Metchnikoff demonstrated that cells also contribute to the immune state of an animal. He observed that certain white blood cells, which he termed phagocytes,were able to ingest (phagocytose) microorganisms and other foreign material. Noting that these phagocytic cells were more active in animals that had been immunized, Metchnikoff hypothesized that cells, rather than serum components, were the major effectors of immunity. The active phagocytic cells identified by Metchnikoff were likely blood monocytes and neutrophils.
Humoral theory of immunity stated that the active immune agents were soluble components (molecules) found in the organism’s “humors” (– соки) rather than its cells. In 1891, Paul Ehrlich used term "antibody" refers to blood antimicrobials. Humoral theory is held, among others, by Robert Koch and Emil von Behring,
In due course (должным образом), a controversy (споры) developed between those who held to the concept of humoral immunity and those who agreed with Metchnikoff’s concept of cell-mediated immunity. They lasted about 30 years. It was later shown that both are correct – immunity requires both cellular and humoral responses. It was difficult to study the activities of immune cells before the development of modern tissue culture techniques, whereas studies with serum took advantage of the ready availability of blood and established biochemical techniques. Because of these technical problems, information about cellular immunity lagged behind findings that concerned humoral immunity.
In 1908 the both – Elie Metchnikoff and Paul Ehrlich – won the Nobel Prize.
Initially, a different serum component was thought to be responsible for each activity (antitoxin, precipitin, and agglutinin), but during the 1930s, mainly through the efforts of Elvin Kabat, a fraction of serum first called gamma-globulin (now immunoglobulin) was shown to be responsible for all these activities. The active molecules in the immunoglobulin fraction are called antibodies.Because immunity was mediated by antibodies contained in body fluids (known at the time as humors), it was called humoral immunity.
In a key experiment in the 1940s, Merrill Chase succeeded in transferring immunity against the tuberculosis organism by transferring white blood cells between guinea pigs. This demonstration helped to rekindle (разжечь) interest in cellular immunity. With the emergence (появление) of improved cell culture techniques in the 1950s, the lymphocytewas identified as the cell responsible for both cellular and humoral immunity. Soon thereafter, experiments with chickens pioneered by Bruce Glick at Mississippi State University indicated that there were two types of lymphocytes: T lymphocytes derived from the thymus mediated cellular immunity, and B lymphocytes from the bursa of Fabricius (an outgrowth of the cloaca in birds) were involved in humoral immunity. The controversy about the roles of humoral and cellular immunity was resolved when the two systems were shown to be intertwined, and that both systems were necessary for the immune response.
By the end of the 1940s vaccines against dangerous infectious pathogens (smallpox, rabies, cholera, plague, typhoid, yellow fever, diphtheria, tetanus – оспы, бешенства, холеры, чумы, брюшного тифа, желтой лихорадки, дифтерии, столбняка) were created. This was era of infectious immunology and vaccination.
3. Early theories attempted to explain the specificity of the antibody–antigen interaction
One of the greatest enigmas (загадки) facing early immunologists was the specificity of the antibody molecule for foreign material, or antigen(the general term for a substance that binds with a specific antibody). Around 1900, Jules Bordet at the Pasteur Institute expanded the concept of immunity by demonstrating specific immune reactivity to nonpathogenic substances, such as red blood cells from other species. Serum from an animal inoculated previously with material that did not cause infection would react with this material in a specific manner, and this reactivity could be passed to other animals by transferring serum from the first. In 1900, Austrian physician-immunologist Karl Landsteiner discovered human blood groups, for which in 1930 was awarded the Nobel Prize. The work of Karl Landsteiner and coworkers showed that injecting an animal with almost any organic chemical could induce production of antibodies that would bind specifically to the chemical. These studies demonstrated that antibodies have a capacity for an almost unlimited range of reactivity, including responses to compounds that had only recently been synthesized in the laboratory and had not previously existed in nature. In addition, it was shown that molecules differing in the smallest detail could be distinguished by their reactivity with different antibodies. Two major theories were proposed to account for this specificity: the selective theory and the instructional theory.
The earliest conception of the selective theory dates to Paul Ehrlich in 1900. Ehrlich said that the main feature of antibodies is their pronounced (выраженный) specificity. In trying to understand specificity phenomenon Ehrlich theorized "side chains", in accordance with which, the antibody are preceded as a receptor on the cell surface. In an attempt to explain the origin of serum antibody, Ehrlich proposed that cells in the blood expressed a variety of receptors, which he called “side-chain receptors,” that could react with infectious agents and inactivate them. Borrowing a concept used by Emil Fischer in 1894 to explain the interaction between an enzyme and its substrate, Ehrlich proposed that binding of the receptor to an infectious agent was like the fit between a lock and key. Ehrlich suggested that interaction between an infectious agent and a cell-bound receptor would induce the cell to produce and release more receptors with the same specificity. According to Ehrlich’s theory, the specificity of the receptor was determined before its exposure to antigen, and the antigen selected the appropriate receptor. Ultimately all aspects of Ehrlich’s theory would be proven correct with the minor exception that the “receptor” exists as both a soluble antibody molecule and as a cell-bound receptor; it is the soluble form that is secreted rather than the bound form released.
In the 1930s and 1940s, the selective theory was challenged by various instructional theories, in which antigen played a central role in determining the specificity of the antibody molecule. According to the instructional theories, a particular antigen would serve as a template around which antibody would fold. The antibody molecule would thereby assume a configuration complementary to that of the antigen template. This concept was first postulated by Friedrich Breinl and Felix Haurowitz about 1930 and redefined in the 1940s in terms of protein folding by Linus Pauling. The instructional theories were formally disproved in the 1960s, by which time information was emerging about the structure of DNA, RNA, and protein that would offer new insights into the vexing problem of how an individual could make antibodies against almost anything.
In the 1950s, selective theories resurfaced as a result of new experimental data and, through the insights of Niels Jerne, David Talmadge, and F. Macfarlane Burnet, were refined into a theory that came to be known as the clonalselection theory.According to this theory, an individual lymphocyte expresses membrane receptors that are specific for a distinct antigen. This unique receptor specificity is determined before the lymphocyte is exposed to the antigen. Binding of antigen to its specific receptor activates the cell, causing it to proliferate into a clone of cells that have the same immunologic specificity as the parent cell. The clonalselection theory has been further refined and is now accepted as the underlying paradigm of modern immunology.
4. The immune system includes innate and adaptive components
Immunity has both a less specific and a more specific component (Fig. ).
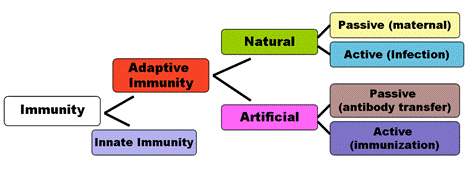
Fig. Components of immunity
The less specific component, innate immunity,provides the first line of defense against infection. Most components of innate immunity are present before the onset (атака) of infection and constitute a set of disease-resistance mechanisms that are not specific to a particular pathogen but that include cellular and molecular components that recognize classes of molecules peculiar to frequently encountered pathogens. Phagocytic cells, such as macrophages and neutrophils, barriers such as skin, and a variety of antimicrobial compounds synthesized by the host all play important roles in innate immunity. In contrast to the broad reactivity of the innate immune system, which is uniform in all members of a species, the specific component, adaptive immunity,does not come into play until there is an antigenic challenge to the organism. Adaptive immunity responds to the challenge with a high degree of specificity as well as the remarkable property of “memory.” Typically, there is an adaptive immune response against an antigen within five or six days after the initial exposure to that antigen. Exposure to the same antigen sometime in the future results in a memory response: the immune response to the second challenge occurs more quickly than the first, is stronger, and is often more effective in neutralizing and clearing the pathogen. The major agents of adaptive immunity are lymphocytes and the antibodies and other molecules they produce.
Because adaptive immune responses require some time to the action bigining, innate immunity provides the first line of defense during the critical period just after the host’s exposure to a pathogen. In general, most of the microorganisms encountered by a healthy individual are readily cleared within a few days by defense mechanisms of the innate immune system before they activate the adaptive immune system.
Дата добавления: 2016-07-18; просмотров: 2221;











