OXIDATION AND REDUCTION REACTIONS
A parallel and independent method of characterizing organic reactions is by oxidation-reduction terminology. Carbon atoms may have any oxidation state from -4 (e.g. CH4 ) to +4 (e.g. C02 ), depending upon their substituents. Fortunately, we need not determine the absolute oxidation state of each carbon atom in a molecule, but only the change in oxidation stateof those carbons involved in a chemical transformation. To determine whether a carbon atom has undergone a redox change during a reaction we simply note any changes in the number of bonds to hydrogen and the number of bonds to more electronegative atoms such as 0, N, F, CI, Br, I, & S that has occurred. Bonds to other carbon atoms are ignored. This count should be conducted for each carbon atom undergoing any change during a reaction.
If the number of hydrogen atoms bonded to a carbon increases, and/or if the number of bonds to more electronegative atoms decreases, the carbon in question has been reduced(i.e. it is in a lower oxidation state).
If the number of hydrogen atoms bonded to a carbon decreases, and/or if the number of bonds to more electronegative atoms increases, the carbon in question has been oxidized (i.e. it is in a higher oxidation state).
If there has been no change in the number of such bonds, then the carbon in question has not changed its oxidation state. In the hydrolysis reaction of a nitrile, the carbon has not changed its oxidation state.
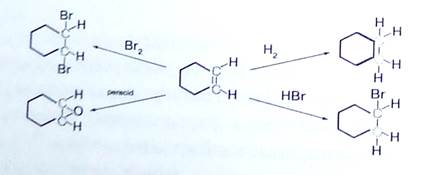
These rules are illustrated by the following four addition reactions involving the same starting material, cyclohexene. Carbon atoms colored blue are reduced, and those colored red are oxidized. In the addition of hydrogen both carbon atoms are reduced, and the overall reaction is termed a reduction. Peracid epoxidation and addition of bromine oxidize both carbon atoms, so these are termed oxidation reactions. Addition of HBr reduces one of the double bond carbon atoms and oxidizes the other; consequently, there is no overall redox change in the substrate molecule.
Since metals such as lithium and magnesium are less electronegative than hydrogen, their covalent bonds to carbon are polarized so that the carbon is negative (reduced) and the metal is positive (oxidized). In the following equation and half-reactions the carbon atom (blue) is reduced and the magnesium (magenta) is oxidized.
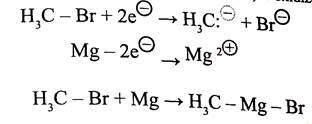
Дата добавления: 2017-10-04; просмотров: 1297;











