Types of Chemical Reactions
From the kinetic stand point, the reactions are classified into two groups:
· Homogeneous reactions which occur in one phase only. It may be a gaseous phase or a liquid phase.
· Heterogeneous reactions which take place in two or more phases, e.g., gaseous reactions taking place on the surface of a solid catalyst or on the walls of the container.
Different chemical reactions occur at different rates. On the basis of rates, the chemical reactions are broadly divided into three categories:
1) Very Fast or Instantaneous Reactions: these reactions are so fast that they occur as soon as the reactants are bought together. Generally, these reactions involve ionic species and thus known as ionic reactions. These reactions take about 10-14 to 10-16 seconds for completion. It is almost impossible to determine the rates of these reactions. Some such examples are:
· Precipitation of AgCl when solutions of silver nitrate and sodium chloride are mixed.
AgNO3 + NaCl → AgCl + NaNO3
· Precipitation of BaSO4 when solutions of barium chloride and sulphuric acid and mixed.
BaCl2 + H2SO4 → BaSO4 + 2HCl
· Neutralisation of an acid with a base when their aqueous solutions are mixed.
HCl + NaOH → NaCl + H2O
2) Very Slow Reactions: there are certain reactions which are extremely slow. They make take months together to show any measurable change at room temperature. It is also difficult to study the kinetics of such reactions. Some examples are:
· Reaction between hydrogen and oxygen at room temperature
· Reaction of atmospheric H2S on basic lead acetate.
· Reaction between carbon and oxygen: carbon and oxygen are thermodynamically less stable than CO2 at 298 K, yet coke does not spontaneously catch fire in air and remains unreacted even for years.
· Reaction between carbon monoxide and hydrogen: The reaction is thermodynamically feasible at 298 K bur in actual practice the reaction proceeds infinitesimally slowly.
3) Moderate Reactions:Between the above two extremes, there are a number of reactions which take place at moderate and measurable rates at room temperature and it is these reactions which are studied in chemical kinetics. Mostly these reactions are molecular in nature. Some common examples of such type are given below:
· Decomposition of hydrogen peroxide: 2H2O2 → 2HO + O2
· Decomposition of nitrogen pentoxide: 2N2O5 → 2N2O4 + O2
· Hydrolysis of an ester: CH3COOC2H5 + NaOH → CH3COONa + C2H5OH
· Inversion of cane sugar in aqueous solution:
C12H22O11 + H2O → C6H12O6 + C6H12O6
· Reaction between nitrogen dioxide and carbon monoxide:
NO2 + CO → NO + CO2
· Reaction between ferric chloride and stannous chloride:
2FeCl3(aq) + SnCl2(aq) → 2FeCl2(aq) + SnCl4(aq)
· Decolourisation of acidified potassium permanganate with sodium oxalate.
· Reaction between nitric oxide and chlorine: NO + Cl2 → NOCl2
The chemical reactions can be slowed down or speeded up by changing conditions under which they occur. For example, very slow reaction. CO + 2H2 → CH3OH, can be speeded up by maintaining temperature around 400oC, pressure about 300 atmosphere and using a catalyst containing ZnO and Cr2O3. The decay of food articles can be slowed down by reserving them in refrigerators.
It is possible to classify chemical reactions into single (having only one stoichiometric equation) and multiple (having more than one stoichiometric equation) reaction types. The latter type many be further sub-divided into four different sub-types.
| Single reaction: | A + B ® C | |
| Multiple reactions: | A ® B ® C | Series or consecutive |
| A ® B ¯ C | Competitive parallel | |
| A ® B R ® S | Side-by-side parallel | |
| A + B ® R R + B ® S | Series/parallel |
In the cases of multiple reactions, it is important to bear in mind two points:
1) as well as having multiple stoichiometric equations, these reaction types also have corresponding multiple reaction rate expressions; and, 2) since there is more than one product which can be formed, the additional issue of desired product optimisation must be taken into consideration when designing reactor systems and choosing operating conditions for these types of reactions.
Reaction Rate
Thus, chemical kinetics deals with the study of reaction rate. Every chemical reaction occurs at a definite rate under a given set of conditions. Some reactions are very fast and some other reactions are comparatively slow. Reaction rate can be defined as, "The change in the concentration of a reactant or product per unit time and per unit volume (for homogeneous reactions) or per unit area (for heterogeneous reactions)":
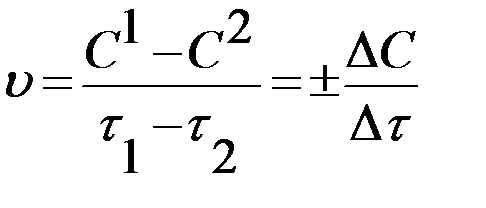
where DC is change in the concentration of reactant or product during time interval Dt.
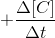 = rate at which concentration of product increases
= rate at which concentration of product increases
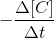 = rate at which concentration of reactant decreases
= rate at which concentration of reactant decreases
Thus, chemical reaction speed is the reverse quantity of the reaction time. At certain conditions, the rates are functions of concentrations. Depending on the time interval between measurements, the rates are called:
· average rate: rate measured between long time interval
· instantaneous rate: rate measured between very short interval
· initial rate: instantaneous rate at the beginning of an experiment
However, a more realistic representation for a reaction rate is the change in concentration per unit time, either the decrease of concentration per unit time of a reactant or the increase of concentration per unit time of a product. In this case, the rate is expressed in mol/l×sec. For the reaction to be useful, either in the laboratory or in nature, it must occur at a reasonable rate.
Rate of reaction is not uniform. It goes on decreasing from moment to moment due to decrease in the concentration(s) of reactant(s) with the progress of reaction i.e. with time as shown below by rate vs time curve. Thus, the rate defined above is actually the average rate of reaction during the time interval considered.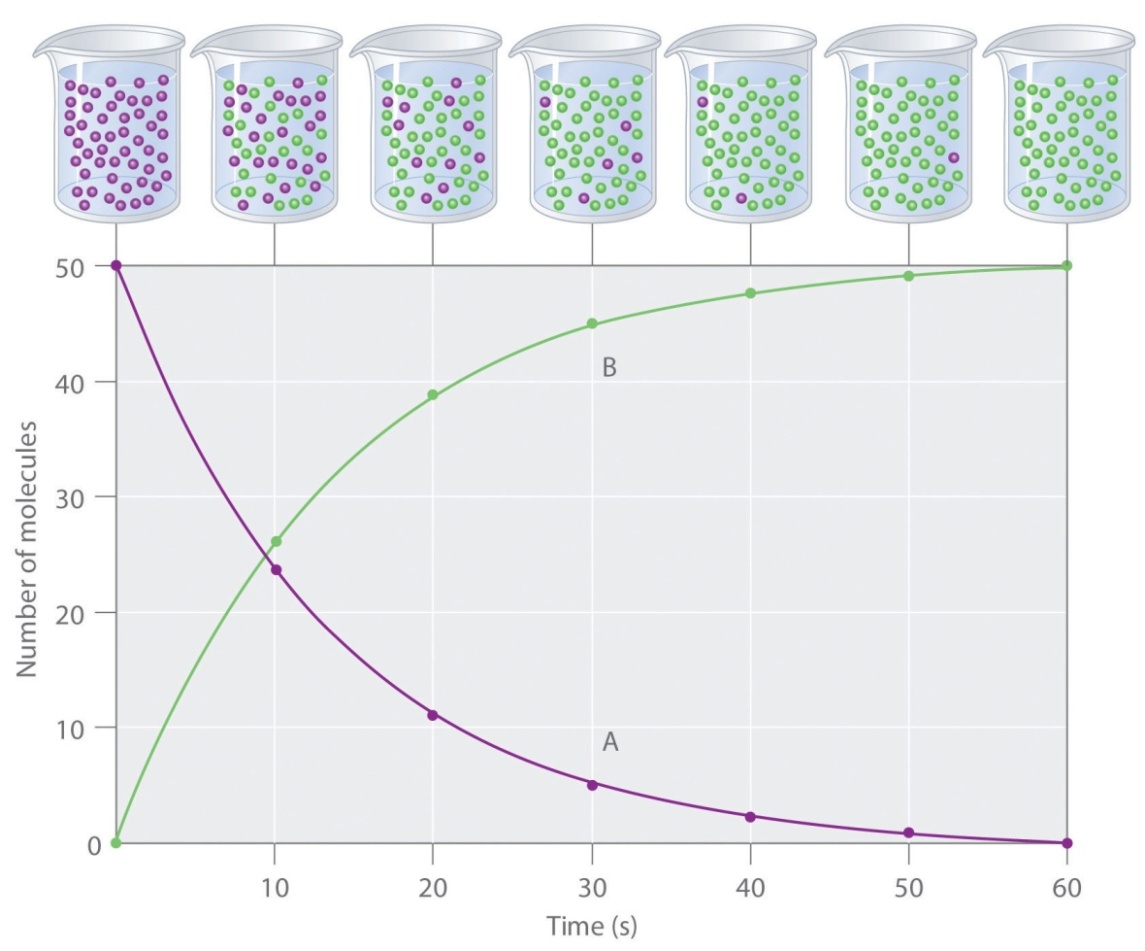
Дата добавления: 2017-05-02; просмотров: 1485;











