Antibody-Antigen Interactions and the Function of the Fab
The Structure of Immunoglobulins
A basic immunoglobulin (Ig) molecule contains four polypeptide chains connected by disulfide bonds. For example, in IgG moleculetwo functionally distinct segments called fragments can be differentiated. The two “arms” that bind antigen are termed antigen binding fragments (Fabs),and the rest of the molecule is the crystallizable fragment (Fc),so called because it has been crystallized in pure form. The distal end of each Fab fragment (consisting of the variable regions of the heavy and light chains) folds into a groove that will accommodate one antigenic determinant. The presence of a special hinge region at the site of attachment between the Fab and Fc fragments allows swiveling of the Fab fragments. In this way, they can change their angle to accommodate nearby antigen sites that vary slightly in distance and position. The Fc fragment is involved in binding to various cells and molecules of the immune system itself. Figure 1 shows three views of antibody structure.
Antibody-Antigen Interactions and the Function of the Fab
The site on the antibody where the antigenic determinant inserts is composed of a hypervariable region whose amino acid content can be extremely varied. Antibodies differ somewhat in the exactness of this groove for antigen, but a certain complementary fit is necessary for the antigen to be held effectively (figure 2). So specific are some immunoglobulins for antigen that they can distinguish between a single functional group of a few atoms. Because the specificity of the Fab sites is identical, an Ig molecule can bind antigenic determinants on the same cell or on two separate cells and thereby link them.
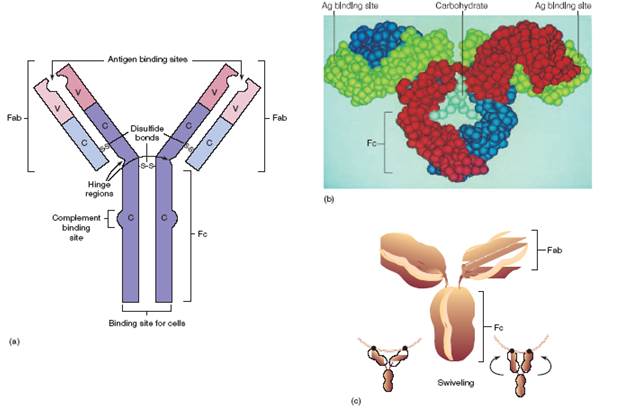
FIGURE 1 Working models of antibody structure. (a)Diagrammatic view of IgG depicts the principal functional areas (Fabs and Fc) of the molecule. (b)Realistic model of immunoglobulin shows the tertiary and quaternary structure achieved by additional intrachain and interchain bonds and theposition of the carbohydrate component. (c)The “peanut” model of IgG helps illustrate swiveling of Fabs relative to one another and to Fc.
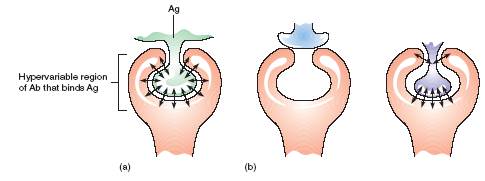
FIGURE 2 Antigen-antibody binding.The union of antibody (Ab) and antigen (Ag) is characterized by a certain degree of fit and is supported by weak linkagessuch as hydrogen bonds and electrostatic attraction. (a)In a snug fit such as that shown here, there is great opportunity for attraction and strongattachment. The strength of this union confers high affinity. (b)Examples of the relationship of other antigens with this same antibody. The first Ag clearlycannot be accommodated. The second (purple) antigen is not a perfect fit, but it can bind to the antibody.
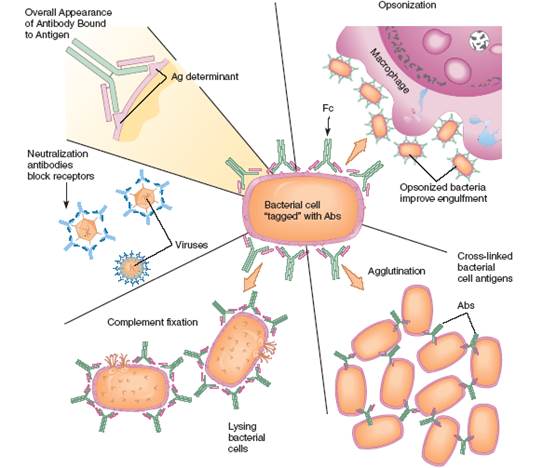
The principal activity of an antibody is to unite with, immobilize, call attention to, or neutralize the antigen for which it was formed (figure 3). Antibodies called opsoninsstimulate opsonization,a process in which microorganisms or other particles are coated with specific antibodies so that they will be more readily recognized by phagocytes, which dispose of them. Opsonization has been likened to putting handles on a slippery object to provide phagocytes a better grip. The capacity for antibodies to aggregate, or agglutinate, antigens is the consequence of their cross-linking cells or particles into large clumps. The interaction of an antibody with complement can result in the specific rupturing of cells and some viruses. In neutralizationreactions, antibodies fill the surface receptors on a virus or the active site on a molecule to prevent it from functioning normally. Antitoxinsare a special type of antibody that neutralize bacterial exotoxins. But not all antibodies are protective; some neither benefit nor harm, and a few actually cause autoimmune diseases.
Дата добавления: 2016-07-18; просмотров: 2978;











