Plant, Animal and Bacterial Cells
When the individual cells of plants and animals (Fig. 1.3) are compared, they have many characteristics in common. Cells are the basic structural and functional units of both plants and animals. They share many characteristics: both are multicellular organisms; all plant and animal cells are eukaryotic; and most organelles are present in both types of cells. Some characteristics, however, are unique to the cells of each type of an organism.
| Fluid-filled pinocytic vesicle Centriole Small vacuole Smooth endoplasmic reticulum Lysosome | 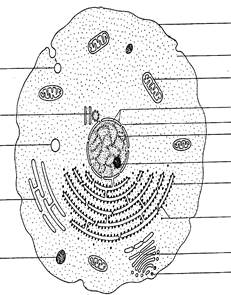
| Cell membrane Cytoplasm Mitochondrion Nuclear membrane Pore in membrane Nucleus Nucleolus Rough endoplasmic reticulum Ribosomes Golgi apparatus Secretory vesicle |
| Fig. 1.3. Ultrastructure of generalised animal cell |
Plant cells may contain three structures not found in animal cells: cell walls, large central vacuoles, and plastids are characteristic. Centrioles are found in some but not all types of plant and animal cells. Bacteria and blue-green bacteria are quite different from other cells. They have fewer structures than plant or animal cells; they carry out all of the life processes that other cells carry out. A bacterium has a cell wall, a cell membrane and cytoplasm. But, there is no nucleus, nuclear membrane, endoplasmic reticulum or mitochondrium. The chromosome material, which directs the cell’s activities, floats freely through the cytoplasm.
1.2. Energy and Energy Conversions.
Chemistry aspects of life organisation
Physicists define energyas the capacity to do work which occurs when a force operates on an object over a distance. In biochemistry, it is more useful to consider energy as the capacity for change. No cell creates energy—all living things must obtain energy from the environment. Indeed, one of the fundamental physical laws is that energy can neither be created nor destroyed. However, energy can be transformed from one type into another and living cells carry out many such energy transformations. Energy transformations are linked to the chemical transformations that occur in cells—the breaking of chemical bonds, the movement of substances across membranes and so forth. Energy changes are related to changes in matter. Energy comes in many forms, such as chemical energy, light energy and mechanical energy. But all forms of energy can be considered as one of two basic types:
Kinetic energyis the energy of movement. This type of energy does work that alters the state or motion of matter. It can exist in the form of heat, light, electric energy and mechanical energy among others.
Potential energyis the energy of state or position—that is, stored energy. It can be stored in chemical bonds, as a concentration gradient and as electric potential among other ways. Water stored behind a dam has potential energy. When the water is released from the dam, some of this potential energy is converted into kinetic energy which can be harnessed to do work.
Likewise, fatty acids store chemical energy in their C—H bonds and C—C bonds and that energy can be released to do biochemical work. Most of living beings consist of cells. In all cells of all organisms, two types of metabolic reactions occur:
Anabolic reactions(anabolism) link together simple molecules to form more complex molecules. The synthesis of a protein from amino acids is an anabolic reaction. Anabolic reactions require an input of energy and capture it in the chemical bonds that are formed.
Catabolic reactions(catabolism) break down complex molecules into simpler ones and release the energy stored in chemical bonds.
Catabolic and anabolic reactions are often linked. The energy released in catabolic reactions is used to drive anabolic reactions—that is, to do biological work. Cellular activities such as growth, movement and active transport of ions across a membrane all require energy and none of them would proceed without a source of energy. In the discussion that follows, will be discovered the physical laws that govern all energy transformations, identified the energy available to do biological work and considered the direction of energy flow. All these processes can be explained by laws of thermodynamics.
The first law: Energy is neither created nor destroyed
Energy can be converted from one form to another. For example, by striking a match, you convert potential chemical energy to light and heat. The first law of thermodynamics states that in any such conversion of energy, it is neither created nor destroyed.
The first law tells us that in any conversion of energy from one form to another, the total energy before and after the conversion is the same. Potential energy in the chemical bonds of carbohydrates and lipids can be converted to potential energy in the form of ATP. This energy can then be used to produce potential energy in the form of concentration gradients established by active transport and can be converted to kinetic energy and used to do mechanical work, such as muscle contraction.
The second law: Not all energy can be used and disorder tends to increase
The second law of thermodynamics states that, although energy cannot be created or destroyed, when energy is converted from one form to another, some of that energy becomes unavailable to do work. In other words, no physical process or chemical reaction is 100 percent efficient and not all the energy released can be converted to work. Some energy is lost to a form associated with disorder. The second law applies to all energy transformations but we will focus here on chemical reactions in living systems.
Not all energy can be used
In any system, the total energy includes the usable energy that can do work and the unusable energy that is lost to disorder: total energy = usable energy + unusable energy.
In biological systems, the total energy is called enthalpy(H). The usable energy that can do work is called free energy(G). Free energy is what cells require for all the chemical reactions of cell growth, cell division and the maintenance of cell health. The unusable energy is represented by entropy(S) which is a measure of the disorder of the system multiplied by the absolute temperature (T). Thus, it is possible to rewrite the word equation above more precisely as H = G + TS,
Or, if rearrange this expression, G = H – TS (usable energy).
Although G, H or S cannot be measured absolutely, it is possible to determine the change in each at a constant temperature. Such energy changes are measured in calories (cal) or joules (J). A change in energy is represented by the Greek letter delta (D). For example, the change in free energy (DG) of any chemical reaction is equal to the difference in free energy between the products and the reactants, DGreaction = Gproducts – Greactants.
If the necessary free energy is not available, the reaction does not occur.
Depending on the sign and magnitude of DS, the entire term, T x S, may be negative or positive, large or small. In other words, in living systems at a constant temperature (no change in T), the magnitude and sign of DG can depend a lot on changes in entropy. Large changes in entropy make DG more negative in value, as it is shown by the negative sign in front of the T x S term. If a chemical reaction increases entropy, its products are more disordered or random than its reactants. If there are more products than reactants, as in the hydrolysis of a protein to its amino acids, the products have considerable freedom to move around. The disorder in a solution of amino acids will be large, compared with that in the protein in which peptide bonds and other forces prevent free movement. Thus, in hydrolysis, the change in entropy (DS) will be positive. If there are fewer products and they are more restrained in their movements than the reactants, DS will be negative.
For example, a large protein linked by peptide bonds is less free in its movements than a solution of the hundreds or thousands of amino acids from which it was synthesized.
Disorder tends to increase
The second law of thermodynamics also predicts that, as a result of energy conversions, disorder tends to increase. Chemical changes, physical changes and biological processes all tend to increase entropy and therefore tend toward disorder or randomness. This tendency for disorder to increase gives a directionality to physical processes and chemical reactions. It explains why some reactions proceed in one direction rather than another. How does the second law apply to organisms? Consider the human body with its highly complex structures constructed of simpler molecules. This increase in complexity is in apparent disagreement with the second law. But this is not the case. Constructing 1 kg of a human body requires that about 10 kg of biological materials be metabolized and in the process converted to CO2, H2O and other simple molecules, and these conversions require a lot of energy. This metabolism creates far more disorder than the order in 1 kg of flesh.
Life requires a constant input of energy to maintain order
There is no disagreement with the second law of thermodynamics. Having seen that the physical laws of energy apply to living things, it will be now considered how these laws apply to biochemical reactions.
Chemical reactions release or take up energy
In cells, anabolic reactions may make a single product, such as a protein (a highly ordered substance), out of many smaller reactants, such as amino acids (less ordered). Such reactions require or consume energy. Catabolic reactions may break down an ordered reactant, such as a glucose molecule, into smaller, more randomly distributed products, such as carbon dioxide and water. Such reactions give off energy. In other words, some reactions release free energy and others take it up. The amount of energy released (–DG) or taken up (+DG) by a reaction is related directly to the tendency of the reaction to run to completion (the point at which all the reactants are converted to products). Some reactions tend to run toward completion without any input of energy. These reactions, which release free energy, are said to be exergonicand have a negative DG. Reactions that proceed toward completion only with the addition of free energy from the environment are endergonic and have a positive DG. If a reaction runs exergonically in one direction (from reactant A to product B, for example), then the reverse reaction (B to A) requires a steady supply of energy to drive it. If A→B is exergonic (DG < 0), then B →A is endergonic (DG > 0). In principle, chemical reactions can run both forward and backward. For example, if a compound A can be converted into a compound B (A→B), then B, in principle, can be converted into A (B →A), although in given concentrations of A and B, only one of these directions will be favored. Think of the overall reaction as resulting from competition between forward and reverse reactions (A~B). Increasing the concentration of the reactants (A) speeds up the forward reaction and increasing the concentration of the products (B) favors the reverse reaction. At some concentration of A and B, the forward and reverse reactions take place at the same rate. At this concentration, no further net change in the system is observable, although individual molecules are still forming and breaking apart. This balance between forward and reverse reactions is known as chemical equilibrium. Chemical equilibrium is a static state, a state of no net change and a state in which DG = 0.
There is good reason to believe that life, as it is known to be, cannot exist without water. Animals and plants that live on the Earth’s land masses had to evolve elaborate ways to retain the water that makes up about 70 percent of their bodies. Aquatic organisms living in water do not need these water-retention mechanisms; thus, biologists have concluded that the first living things originated in a watery environment. This environment need not have been the lakes, rivers and oceans with which we are familiar. Living organisms have been found in hot springs at temperatures above the usual boiling point of water, in a lake beneath the frozen Antarctic ice, in water trapped 2 miles below the Earth’s surface, in water 3 miles below the surface of the sea, in extremely acid and extremely salty water, and even in the water that cools the interiors of nuclear reactors. With 20 trillion galaxies in the universe, each with 100 billion stars, there are many planets out there and if our own solar system is typical, some of them have the water needed for life. As biologists contemplate how life could originate from nonliving matter, their attention focuses not just on the presence of water but on what is dissolved in it. A major discovery of biology is that living things are composed of the same types of chemical elements as the vast nonliving portion of the universe. This mechanistic view— that life is chemically based and obeys universal physicochemical laws—is a relatively recent one in human history. The concept of a “vital force” responsible for life, different from the forces found in physics and chemistry, was common in Western culture until the nineteenth century and many people still assume such a force exists. However, most scientists adhere to a mechanistic view of life and it is the cornerstone of medicine and agriculture. Before describing how chemical elements are arranged in living creatures, some fundamental chemical concepts on changes of matter will be considered. In addition to changes in state (solid to liquid to gas), substances undergo changes that transform both their composition and their characteristic properties.
The first chemical signatures indicating the presence of life here are about 4 billion years old. Thus, it took 600 million years, during a geological time frame called the Hadean, for the chemical conditions on Earth to become just right for life. Key among those conditions was the presence of water. The Ancient Earth probably had a lot of water high in the atmosphere. But the new planet was hot and this water evaporated into space. As the Earth cooled, it became possible for water to remain on its surface but where did that water come from? One current view is that comets—loose agglomerations of dust and ice that have orbited the sun since the planets formed—struck the Earth repeatedly and brought not only water but other chemical components of life, such as nitrogen. As the Earth cooled, chemicals from the rocks dissolved in the water and simple chemical reactions took place. Some of these reactions could have led to life but impacts of large comets and rocky meteorites would have released enough energy to heat the developing oceans almost to boiling, thus destroying any early life. These large impacts eventually subsided and life gained a foothold about 3.8 to 4 billion years ago. The prebiotic Hadean was over. The Archean had begun and there has been life on the Earth ever since. Like the rest of the chemical world, living things are made up of atoms and molecules.
1.2.1. Life and Chemistry: Atoms and Small Molecules
Atoms: The Constituents of Matter
More than a trillion (1012) atoms could fit over the period at the end of this sentence. Each atom consists of a dense, positively charged nucleus, around which one or more negatively charged electronsmove The nucleus contains one or more protonsand may contain one or more neutrons. Atoms and their component particles have volume and mass, which are properties of all matter. Massmeasures the quantity of matter present; the greater the mass, the greater the quantity of matter. The mass of a proton serves as a standard unit of measure: the atomic mass unit (amu) or dalton (named after the English chemist John Dalton). A single proton or neutron has a mass of about 1 dalton (Da). Because the mass of an electron is negligible compare to the mass of a proton or a neutron, the contribution of electrons to the mass of an atom can usually be ignored when measurements and calculations are made.
These are electrons, however, that determine how atoms will interact in chemical reactions and they will be discussed extensively later in this chapter. Each proton has a positive electric charge defined as +1 unit of charge. An electron has a negative charge equal and opposite to that of a proton; thus, the charge of an electron is –1 unit. The neutron, as its name suggests, is electrically neutral, so its charge is 0 unit. Unlike charges (+/–) attract each other; like charges (+/+ or –/–) repel each other. Atoms are electrically neutral: the number of protons in an atom equals the number of electrons.
An element is made up of only one kind of atom
An elementis a pure substance that contains only one type of atom. The element hydrogen consists only of hydrogen atoms; the element iron consists only of iron atoms. The atoms of each element have certain characteristics or properties that distinguish them from the atoms of other elements. The more than 100 elements, found in the universe, are arranged in the periodic table. These elements are not found in equal amounts. Stars have abundant hydrogen and helium. The earth’s crust and those of the neighboring planets are almost half oxygen, 28 percent silicon, 8 percent aluminum, 2–5 percent each of sodium, magnesium, potassium, calcium and iron, and contain much smaller amounts of the other elements. About 98 percent of the mass of every living organism (bacterium, turnip or human) is composed of just six elements: carbon, hydrogen, nitrogen, oxygen, phosphorus and sulfur. The chemistry of these six elements will be the primary concern here but the others are not unimportant. Sodium and potassium, for example, are essential for nerves to function; calcium can act as a biological signal; iodine is a component of a vital hormone; and plants need magnesium as part of their green pigment (chlorophyll) and molybdenum in order to incorporate nitrogen into biologically useful substances.
The number of protons identifies the element
An element is distinguished from other elements by the number of protons in each of its atoms. This number which does not change, is called the atomic number. An atom of helium has 2 protons, and an atom of oxygen has 8 protons; the atomic numbers of these elements are thus 2 and 8, respectively. Along with a definitive number of protons, every element except hydrogen has one or more neutrons in its nucleus. The mass number of an atom is the total number of protons and neutrons in its nucleus. The nucleus of a helium atom contains 2 protons and 2 neutrons; oxygen has 8 protons and 8 neutrons. Therefore, helium has a mass number of 4 and oxygen a mass number of 16. The mass number may be thought of as the mass of the atom in daltons. Each element has its own one- or two-letter chemical symbol. For example, H stands for hydrogen, He for helium and O for oxygen. Some symbols come from other languages: Fe (from the Latin ferrum) stands for iron, Na (Latin natrium) for sodium and W (German Wolfram) for tungsten.
Electron behavior determines chemical bonding
When considering atoms, biologists are concerned primarily with electrons because the behavior of electrons explains how chemical changes occur in living cells. These changes, called chemical reactions or just reactions, are changes in the atomic composition of substances. The characteristic number of electrons in each atom of an element determines how its atoms will react with other atoms. All chemical reactions involve changes in the relationships of electrons with one another.
The location of a given electron in an atom at any given time is impossible to determine. We can only describe a volume of space within the atom where the electron is likely to be. The region of space where the electron is found at least 90 percent of the time is the electron’s orbital. In an atom, a given orbital can be occupied by at most two electrons. Thus, any atom larger than helium (atomic number 2) must have electrons in two or more orbitals. The different orbitals have characteristic forms and orientations in space. The orbitals, in turn, constitute a series of electron shells or energy levels, around the nucleus. The innermost electron shell consists of only one orbital called an s orbital. Hydrogen (1H) has one electron in its first shell; helium (2He) has two of them. All other elements have two first-shell electrons, as well as electrons in other shells. The second shell is made up of four orbitals (an s orbital and three p orbitals) and hence can hold up to eight electrons. The s orbitals fill with electrons first and their electrons have the lowest energy. Subsequent shells have different numbers of orbitals but the outermost shells usually hold only eight electrons. In any atom, the outermost electron shell determines how the atom combines with other atoms—that is, how the atom behaves chemically. When an outermost shell consisting of four orbitals contains eight electrons, there are no unpaired electrons. Such an atom is stable and will not react with other atoms. Examples of chemically stable elements are helium, neon and argon. Reactive atoms seek to attain the stable condition of having no unpaired electrons in their outermost shells. They attain this stability by sharing electrons with other atoms or by gaining or losing one or more electrons. In either case, the atoms are bonded together. Such bonds create stable associations of atoms called molecules.
A moleculeis two or more atoms linked by chemical bonds. The tendency of atoms in stable molecules to have eight electrons in their outermost shells is known as the octet rule. Many atoms in biologically important molecules—for example, carbon (C) and nitrogen (N)—follow the octet rule. However, some biologically important atoms are exceptions to the rule. Hydrogen (H) is the most obvious exception attaining stability when only two electrons occupy its single shell.
1.2.2. Chemical Bonds: Linking Atoms Together
A chemical bondis an attracting force that links two atoms together to form a molecule. There are several kinds of chemical bonds. Firstly covalent bonds will be discussed, the strong bonds that result from the sharing of electrons. Then will be examined other kinds of interactions, including hydrogen bonds that are weaker than covalent bonds but enormously important to biology. Finally, will be considered ionic bonding which is a consequence of the loss or gain of electrons by atoms.
Covalent bonds consist of shared pairs of electrons
When two atoms attain stable electron numbers in their outermost shells by sharing one or more pairs of electrons, a covalent bondforms. Consider two hydrogen atoms in close proximity, each with a single unpaired electron in its outer shell. Each positively charged nucleus attracts the other atom’s unpaired electron but this attraction is balanced by each electron’s attraction to its own nucleus. Thus, two unpaired electrons become shared by both atoms filling the outer shells of both of them. Two atoms are, thus, linked by a covalent bond and a hydrogen gas molecule (H2) is formed. A molecule made up of more than one type of atom is called a compound. A molecular formula uses chemical symbols to identify the different atoms in a compound and subscript numbers to show how many of each type of atoms are present. Thus, the formula for sucrose—table sugar—is C12H22O11. Each compound has a molecular weight(molecular mass) that is the sum of the atomic weights of all atoms in the molecule. Looking at the Periodic table in Annex 3 you can calculate the molecular weight of table sugar to be 342. Molecular weights are usually related to a molecule’s size. A carbon atom has a total of six electrons; two electrons fill its inner shell and four are in its outer shell. Because its outer shell can hold up to eight electrons, carbon can share electrons with up to four other atoms—it can form four covalent bonds. When an atom of carbon reacts with four hydrogen atoms, a molecule called methane (CH4) forms. Thanks to electron sharing, the outer shell of methane’s carbon atom is filled with eight electrons and the outer shell of each hydrogen atom is also filled. Four covalent bonds—each consisting of a shared pair of electrons—hold methane together. Table shows the covalent bonding capacities of some biologically significant elements.
Orientation of covalent bonds
Covalent bonds are very strong. The thermal energy that biological molecules ordinarily have at body temperature is less than 1 percent of that needed to break covalent bonds. Thus biological molecules, most of which are put together with covalent bonds, are quite stable. This means that their three-dimensional structures and the spaces they occupy are also stable. A second property of covalent bonds is that, for a given pair of atoms, they are the same in length, angle and direction, regardless of the larger molecule of which the particular bond is a part. The four filled orbitals around the carbon nucleus of methane, for example, distribute themselves in space so that the bonded hydrogens are directed to the corners of a regular tetrahedron with carbon in the centre. The three-dimensional structure of carbon and hydrogen is the same in a large, complicated protein as it is in the simple methane molecule. This property of covalent bonds makes the prediction of biological structure possible. Although the orientations of orbitals and the shapes of molecules differ, depending on the types of atoms involved and how they are linked together, it is essential to remember that all molecules occupy space and have three-dimensional shapes. The shapes of molecules contribute to their biological functions.
Unequal sharing of electrons.
If two atoms of the same element are covalently bonded, there is an equal sharing of the pair(s) of electrons in the outer shell. However, when two atoms are of different elements, the sharing is not necessarily equal. One nucleus may exert a greater attractive force on the electron pair than the other nucleus, so that the pair tends to be closer to that atom. The attractive force that an atom exerts on electrons is its electronegativity. It depends on how many positive charges a nucleus has (nuclei with more protons are more positive and thus more attractive to electrons) and how far away the electrons are from the nucleus (closer means more electronegativity).
The closer two atoms are in electronegativity, the more equal their sharing of electrons will be. Looking at the table electronegativities of some elements important in biological systems, it is obvious that two oxygen atoms, both with electronegativity of 3.5, will share electrons equally, producing what is called a nonpolar covalent bond. So will do two hydrogen atoms (both 2.1). But when hydrogen bonds with oxygen to form water, the electrons involved are unequally shared: they tend to be nearer to the oxygen nucleus because it is the most electronegative of two. The result is called a polar covalent bond. Because of this unequal sharing of electrons, the oxygen end of the hydrogen–oxygen bond has a slightly negative charge (symbolized D– and spoken as “delta negative,” meaning a partial unit of charge) and the hydrogen end is slightly positive (D+). The bond is polarbecause these opposite charges are separated at the two ends or poles of the bond. The partial charges that result from polar covalent bonds produce polar molecules or polar regions of large molecules. Polar bonds greatly influence the interactions between molecules containing them.
Hydrogen bonds
Hydrogen bonds may form within or between atoms with polar covalent bonds
In liquid water, the negatively charged oxygen atom of one water molecule is attracted to the positively charged hydrogen (D+) atoms of another water molecule. (Remember, negative charges attract positive charges.) The bond resulting from this attraction is called a hydrogen bond. Hydrogen bonds are not restricted to water molecules. They may form between an electronegative atom and a hydrogen atom covalently bonded to a different electronegative atom. A hydrogen bond is a weak bond; it has about one-tenth (10%) the strength of a covalent bond between a hydrogen atom and an oxygen atom. However, where many hydrogen bonds form, they have considerable strength and greatly influence the structure and properties of substances. Hydrogen bonds also play important roles in determining and maintaining the three-dimensional shapes of giant molecules such as DNA and proteins.
Ionic bonds
Ionic bonds form by electrical attraction
When one interacting atom is much more electronegative than the other, a complete transfer of one or more electrons may take place. Consider sodium (electronegativity 0.9) and chlorine (3.1). A sodium atom has only one electron in its outermost shell; this condition is unstable. A chlorine atom has seven electrons in its outer shell—another unstable condition. Since the electronegativities of these elements are so different, any electrons involved in bonding will tend to be much nearer to the chlorine nucleus—so near, in fact, that there is a complete transfer of the electron from one element to the other. This reaction between sodium and chlorine makes the resulting atoms more stable. The result is two ions. Ions are electrically charged particles that form when atoms gain or lose one or more electrons.
The sodium ion (Na+) has a +1 unit charge because it has one less electron than it has protons. The outermost electron shell of the sodium ion is full, with eight electrons, so the ion is stable. Positively charged ions are called cations.
The chloride ion (Cl–) has a –1 unit charge because it has one more electron than it has protons. This additional electron gives Cl– a stable outermost shell with eight electrons. Negatively charged ions are called anions. Some elements form ions with multiple charges by losing or gaining more than one electron. Examples are Ca2+ (calcium ion, created from a calcium atom that has lost two electrons) and Mg2+ (magnesium ion). Two biologically important elements each yield more than one stable ion: Iron yields Fe2+ (ferrous ion) and Fe3+ (ferric ion) and copper yields Cu+ (cuprous ion) and Cu2+ (cupric ion). Groups of covalently bonded atoms that carry an electric charge are called complex ions; examples include NH4+ (ammonium ion), SO42– (sulfate ion), and PO43– (phosphate ion). The charge from an ion radiates from it in all directions. Once formed, ions are usually stable and no more electrons are lost or gained. Ions can form stable bonds resulting in stable solid compounds such as sodium chloride (NaCl) and potassium phosphate (K3PO4).
Ionic bondsare bonds formed by electrical attraction between ions bearing opposite charges. In sodium chloride—familiar to us as table salt—cations and anions are held together by ionic bonds. In solids, the ionic bonds are strong because the ions are close together.
However, when ions are dispersed in water, the distance between them can be large; the strength of their attraction is thus greatly reduced. Under the conditions that exist in the cell, an ionic attraction is less than one-tenth as strong as a covalent bond that shares electrons equally. Not surprisingly, ions with one or more units of charge can interact with polar molecules as well as with other ions.
Such interaction results when table salt or any other ionic solid, dissolves in water: “shells” of water molecules surround the individual ions, separating them. The hydrogen bond described earlier is a type of ionic bond because it is formed by electrical attraction. However, it is weaker than most ionic bonds because it is formed by partial charges (D+ and D–) rather than by whole-unit charges (+1 unit, –1 unit).
Polar and nonpolar substances interact best among themselves
“Like attracts like” is an old saying and nowhere is it more true than in polar and nonpolar molecules which tend to interact with their own kind. Just as water molecules interact with one another through polarity-induced hydrogen bonds, any molecule that is itself polar will interact with other polar molecules by weak (D+ to D–) attractions in hydrogen bonds. If a polar molecule interacts with water in this way, it is called hydrophilic(“water-loving”). What about nonpolar molecules? For example, carbon (electronegativity 2.5) forms nonpolar bonds with hydrogen (electronegativity 2.1). The resulting hydrocarbon molecule— that is, a molecule containing only hydrogen and carbon atoms—is nonpolar, and in water it tends to aggregate with other nonpolar molecules rather than with polar water. Such molecules are known as hydrophobic(“waterhating”) and the interactions between them are called hydrophobic interactions. It is important to realize that hydrophobic substances do not really “hate” water; they can form weak interactions with it (recall that the electronegativities of carbon and hydrogen are not exactly the same). But these interactions are far weaker than the hydrogen bonds between the water molecules and so the nonpolar substances keep to themselves.
1.2.3. Chemical Reactions: Atoms Change Partners
A chemical reactionoccurs when atoms combine or change their bonding partners. Consider the combustion reaction that takes place in the flame of a propane stove. When propane (C3H8) reacts with oxygen gas (O2), the carbon atoms become bonded to oxygen atoms instead of to hydrogen atoms and the hydrogen atoms become bonded to oxygen instead of carbon. As the covalently bonded atoms change partners, the composition of the matter changes and propane and oxygen gas become carbon dioxide and water. This chemical reaction can be represented by the equation
C3H8 + 5 O2 → 3 CO2 + 4 H2O + energy
In this equation, the propane and oxygen are the reactants, and the carbon dioxide and water are the products. In this case, the reaction is complete: all the propane and oxygen are used up in forming two products. The arrow symbolizes the direction of the chemical reaction. The numbers preceding the molecular formulas balance the equation and indicate how many molecules are used or are produced. In this and all other chemical reactions, matter is neither created nor destroyed. The total number of carbons on the left equals the total number of carbons on the right. However, there is another product of this reaction - energy. The heat and light of the stove’s flame reveal that the reaction of propane and oxygen releases a great deal of energy. Energyis defined as the capacity to do work but on a more intuitive level, it can be thought of as the capacity for change. Chemical reactions do not create or destroy energy but changes in energy usually accompany chemical reactions. In the reaction between propane and oxygen, the energy that was released as heat and light was already present in the reactants in another form, called potential chemical energy.
In some chemical reactions, energy must be supplied from the environment (for example, some substances will react only after being heated) and some of this supplied energy is stored as potential chemical energy in the bonds formed in the products. We can measure the energy associated with chemical reactions using a unit called a calorie(cal). A calorie* is the amount of heat energy needed to raise the temperature of 1 gram of pure water from 14.5°C to 15.5°C. Another unit of energy that is increasingly used is the joule (J). Compare data on energy: joules to joules and calories to calories. The two units can be interconverted: 1 J = 0.239 cal, and 1 cal = 4.184 J. Thus, for example, 486 cal = 2,033 J, or 2.033 kJ. Although defined in terms of heat, the calorie and the joule are measures of any form of energy — mechanical, electric, or chemical. Many biological reactions have much in common with the combustion of propane. The fuel is different — it is the sugar glucose, rather than propane — and the reactions proceed by many intermediate steps that permit the energy released from the glucose to be harvested and put to use by the cell. But the products are the same: carbon dioxide and water. These reactions were key to the origin of life from simpler molecules. Energy changes, oxidation– reduction reactions and several other types of chemical reactions that are prevalent in living systems will be presented in the sections that follow.
1.2.4. Water: Structure and Properties
Water can exist in three states: solid (ice), liquid or gas (vapor). Liquid water is probably the medium in which life originated on the Earth and it is in water that life evolved for its first billion years. In this section, will be explored how the structure and interactions of water molecules make water essential to life.
Water has a unique structure and special properties
The water molecule H2O has unique chemical features. As we learned in the preceding sections, water is a polar molecule that can form hydrogen bonds. In addition, the shape of water is a tetrahedron. The four pairs of electrons in the outer shell of oxygen repel one another, producing a tetrahedral shape. These chemical features explain some of the interesting properties of water, such as the ability of ice to float, the melting and freezing temperatures of water, the ability of water to store heat, and the ability of water droplets to form. These properties are described in detail below.
Ice floats
In water’s solid state (ice), individual water molecules are held in place by hydrogen bonds, creating a rigid, crystalline structure in which each water molecule is hydrogen-bonded to four other water molecules. Although the molecules are held firmly in place, they are not as tightly packed as they are in liquid water. In other words, solid water is less dense than liquid water which is why ice floats in water. If ice were to sink in water, as almost all other solids do in their corresponding liquids, ponds and lakes would freeze from the bottom up, becoming solid blocks of ice in winter and killing most of the organisms living in them. Once the whole pond had frozen, its temperature could drop well below the freezing point of water. But, because ice floats, it forms a protective insulating layer on the top of the pond, reducing heat flow to the cold air above. Thus, fish, plants and other organisms in the pond are not subjected to temperatures lower than 0°C, the freezing point of pure water. The recent discovery of liquid water below the polar ice on Mars has created speculation that life could exist in that environment.
Melting and freezing
Water is a moderator of temperature changes. Compared with other nonmetallic substances of the same size, molecular ice requires a great deal of heat energy to melt. In the opposite process — freezing — a great deal of energy is lost when water is transformed from liquid to solid.
Heat storage
Water contributes to the surprising constancy of the temperature found in the oceans and other large bodies of water throughout the year. The temperature changes of coastal land masses are also moderated by large bodies of water. Indeed, water helps minimize variations in atmospheric temperature across the planet. This moderating ability is a result of the high heat capacity of liquid water. The specific heat of a substance is the amount of heat energy required to raise the temperature of 1 gram of that substance by 1°C. Raising the temperature of liquid water takes a relatively large amount of heat because much of the heat energy is used to break the hydrogen bonds that hold the liquid together. Compared with other small molecules that are liquids, water has a high specific heat.
Evaporation and cooling
Water has a high heat of vaporization, which means that a lot of heat is required to change water from its liquid to its gaseous state (the process of evaporation). Once again, much of the heat energy is used to break hydrogen bonds. This heat must be absorbed from the environment in contact with the water. Evaporation, thus, has a cooling effect on the environment — whether a leaf, a forest or an entire land mass. This effect explains why sweating cools the human body: as sweat evaporates off the skin, it uses up some of the adjacent body heat.
Cohesion and surface tension
In liquid water, individual water molecules are free to move about. The hydrogen bonds between the molecules continually form and break. In other words, liquid water has a dynamic structure. On average, every water molecule forms 3.4 hydrogen bonds with other water molecules. This number represents fewer bonds than exist in ice but it is still a high number. These hydrogen bonds explain the cohesive strength of liquid water. This cohesive strength permits narrow columns of water to stretch from the roots to the leaves of trees more than 100 meters high. When water evaporates from the leaves, the entire column moves upward in response to the pull of the molecules at the top. Water also has a high surface tension which means that the surface of liquid water exposed to the air is difficult to puncture. The water molecules in this surface layer are hydrogen-bonded to other water molecules below. The surface tension of water permits a container to be filled slightly above its rim without overflowing and it permits small animals to walk on the surface of water.
Water is the solvent of life
A living organism is over 70 percent water by weight, excluding minerals such as bones. Many substances undergo reactions in this watery environment. Others do not and, thus, form biological structures (such as bones). A solution is produced when a substance (the solute) is dissolved in a liquid (the solvent) such as water (forming an aqueous solution). Many of the important molecules in biological systems are polar and, therefore, are soluble in water.
Reactions that take place in an aqueous solution can be studied in two ways:
– Qualitative analysis deals with substances dissolved in water and the chemical reactions that occur there.
– Quantitative analysis measures concentrations or the amount of a substance in a given amount of solution.
Fundamental to quantitative thinking in chemistry and biology is the mole concept. A moleis the amount of an ion or compound (in grams) whose weight is numerically equal to its molecular weight. Thus, a mole of table sugar (C12H22O11) weighs 342 grams.
1.2.5. Acids, Bases and the pH Scale
When some substances dissolve in water, they release hydrogen ions (H+) which are actually single, positively charged protons. These tiny bits of charged matter can attach to other molecules, and in doing so, change their properties. For example, the protons in acid rain can damage plants, and you are probably familiar with excess stomach acidity that affects digestion. In this section, will be examined the properties of substances that release H+ (called acids) and substances that attach to H+ (called bases). We will distinguish strong and weak acids and bases and provide a quantitative means for stating the concentration of H+ in solutions: the pH scale.
Acids donate H+, bases accept H+
If hydrochloric acid (HCl) is added to water, it dissolves and ionizes, releasing the ions H+ and Cl–: HCl → H+ + Cl–.
Because its H+ concentration has increased, such a solution is acidic. Just like the combustion reaction of propane and oxygen the dissolution of HCl to form its ions is a complete reaction. HCl is therefore called a strong acid.
Acids release H+ ions in solution. HCl is an acid, as is H2SO4 (sulfuric acid). One molecule of sulfuric acid may ionize to yield two H+ and one SO42–. Biological compounds that contain —COOH (the carboxyl group; are also acids (such as acetic acid and pyruvic acid), because —COOH → —COO– + H+ not all acids dissolve fully in water. For example, if acetic acid is added to water, at the end of the reaction, there are not just the two ions but some of the original acid as well. Because the reaction is not complete, acetic acid is a weak acid. Bases accept H+ in solution. Just as with acids, there are strong and weak bases. If NaOH (sodium hydroxide) is added to water, it dissolves and ionizes, releasing OH– and Na+ ions: NaOH →Na+ + OH–. Because the concentration of OH– increases and OH– absorbs H+ to form water, such a solution is basic. Because this reaction is complete, NaOH is a strong base. Weak bases include the bicarbonate ion (HCO3–), which can accept a H+ ion and become carbonic acid (H2CO3), and ammonia (NH3), which can accept a H+ and become an ammonium ion (NH4+). Amino groups (—NH2) in biological molecules can also accept protons, thus, acting as bases: —NH2 + H+ →—NH3+.
The reactions between acids and bases may be reversible
When acetic acid is dissolved in water, two reactions happen. First, the acetic acid forms its ions: CH3COOH → CH3COO– + H+. Then, once ions are formed, they re-form acetic acid:
CH3COO– + H+ → CH3COOH
This pair of reactions is reversible. A reversible reactioncan proceed in either direction—left to right or right to left—depending on the relative starting concentrations of the reactants and products. The formula for a reversible reaction can be written, using a double arrow: CH3COOH ~ CH3COO– + H+. In principle, all chemical reactions are reversible. In terms of acids and bases, there are two types of reactions, depending on the extent of reversibility:
– Ionization of strong acids and bases is virtually irreversible.
– Ionization of weak acids and bases is somewhat reversible. Many of the acid and base groups on large molecules in biological systems are weak.
Water is a weak acid
The water molecule has a slight but significant tendency to ionize into a hydroxide ion (OH–) and a hydrogen ion (H+). Two water molecules participate in this ionization. One of the two molecules “captures” a hydrogen ion from the other, forming a hydroxide ion and a hydronium ion: The hydronium ion is in effect a hydrogen ion bound to a water molecule. For simplicity, biochemists tend to use a modified representation of the ionization of water:
H2O →H+ + OH–. The ionization of water is important to all living creatures. This fact may seem surprising, since only about one water molecule in 500 million is ionized at any given time. But there will be less surprise when the attention is focused on the abundance of water in living systems and the reactive nature of the H+ produced by ionization.
pH is the measure of hydrogen ion concentration
The terms “acidic” and “basic” refer only to solutions. How acidic or basic a solution is depends on the relative concentrations of H+ and OH– ions in it. The terms “acid” and “base” refer to compounds and ions. A compound or ion that is an acid can donate H+; one that is a base can accept H+. A solution with a pH of less than 7 is acidic — it contains more H+ ions than OH– ions. A solution with a pH of 7 is neutral and a solution with a pH value greater than 7 is basic.
Buffers minimize pH change
Some organisms, probably including the earliest forms of life, live in and have adapted to solutions with extremes of pH. Most organisms control the pH of the separate compartments within their cells. The normal pH of human red blood cells, for example, is 7.4, and deviations of even a few tenths of a pH unit can be fatal. The control of pH is made possible in part by buffers: chemical mixtures that maintain a relatively constant pH even when substantial amounts of an acid or base are added. Abuffer is a mixture of a weak acid and its corresponding base—for example, carbonic acid (H2CO3) and bicarbonate ions (HCO3–). If an acid is added to a solution containing this buffer, not all the H+ ions from that acid stay in solution. Instead, many of them combine with the bicarbonate ions to produce more carbonic acid. This reaction uses up some of the H+ ions in the solution and decreases the acidifying effect of the added acid: HCO3– + H+ ~ H2CO3. If a base is added, the reaction essentially reverses. Some of the carbonic acid ionizes to produce bicarbonate ions and more H+, which counteracts some of the added base. In this way, the buffer minimizes the effects of an added acid or base on pH. This is what happens in the blood where this buffering system is important in preventing significant changes in pH that could disrupt the ability of the blood to function in carrying vital O2 to tissues. A given amount of acid or base causes a smaller change in pH in a buffered solution than in an unbuffered one. Buffers illustrate an important chemical principle in reversible reactions called the law of mass action. Addition of a reactant on one side of a reversible system drives the reaction in the direction that uses up that compound. In this case, addition of an acid drives the reaction in one direction; addition of a base drives it in other direction.
Дата добавления: 2016-07-18; просмотров: 2486;











