Molecules Make Up Cells
Chemical composition of a typical cell is: 70% water, 15% proteins, 10% fats, 4% DNA and other molecules, 1% carbohydrates.
So, most of the cell’s structures are made of large molecules called proteins. These molecules are arranged to give each cell its distinctive characteristics.
Proteins are very large molecules composed of long chains of amino acids, which are nitrogen-containing substances. There are 20 different amino acids in proteins, and from these an extremely large variety of different kinds of protein molecules can be synthesized, each of which has a highly specific activity and function in living systems. All proteinsare made up of one or more chains, linked together by peptide bonds (Fig. 1.4). Amino acids contain carbon, hydrogen, and oxygen. All of them also contain nitrogen. Every amino acid has the same “backbone” structure, which consists of a central carbon atom bonded to an amino group (-NH2) and a carboxyl group (-COON). In every amino acid there is also another atom or group of atoms bonded to the central carbon. A hydrogen atom and a side group are also bounded to the same carbon atom. This basic structure is the same in all amino acids. The side group, is different in each kind of amino acid. This side group can be a hydrogen atom, in which case the amino acid is glycine; a CH3 group, in which case the amino acid is alanine (Fig. 1.5) and so on. The side group, depending on the atom or atoms that compose it, may have a positive charge, be polar (with a negative and positive zone), or have no charge at all (in which case it is insoluble in water).
Informational proteins serve as enzymes, as hemoglobin, and as hormones. Some proteins serve as major structural components of living systems.
Enzymes are globular proteins that control the rate of reactions in living systems. These organic catalysts are able to perform this function because the molecule contains an active site onto which the reactants, or substrate, are temporarily bound. The specificity of enzymes is due to the lock-and-key fit of the active site on the enzyme with the substrate molecule.
amino acids
 amino acids become joined together by peptide bonds
amino acids become joined together by peptide bonds
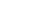 
 hydrogen bonds form between certain amino acids
hydrogen bonds form between certain amino acids
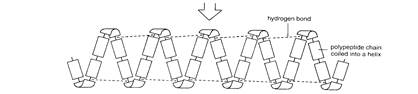
| |||||||||||
| arranged in long parallel strands | folded into spherical shape | folded into spherical shape which incorpo- rates another chemical | |||||||||
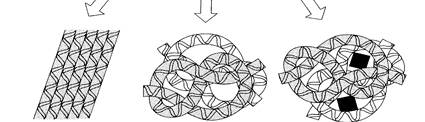
| |||||||||||
| fibrous protein | globular protein | conjugated protein | |||||||||
| Fig. 1.4. Structure of proteins Hemoglobin, the oxygen-carrying molecule of the blood, is composed of four (two pairs) of protein chains each attached to an iron-containing hem group. Substitution of one amino acid for another in one | |||||||||||
| CH3 | |||||||||||

| |||||||||||
| H2N | C | C | OH | ||||||||
| H | O | ||||||||||
Дата добавления: 2016-07-18; просмотров: 1814;