Example: main applied equations for Membrane installation design
Given: Feed water T,oC; QP(G), m3/h, water quality → selection the membrane type; QP:QC = 1:x
Membrane characteristics (from documents): A (surface area, m2); QFmax ≤ a m3/h
4.2.1. Permeability of the membrane (water flux density)
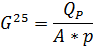
where
Using the nomograms G = f(T), find the real permeability Gt for real temperature of the process, e.g. G10 ≈0,5*G25
4.2.2. Total surface of membrane material to provide QP, m2
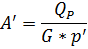
where
4.2.3. The number of membrane elements
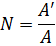
The result should be rounded up to whole number, e.g. 4,5≈5
4.2.4. Actual operating pressure evaluation, kPa
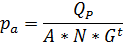
4.2.5. The number of membrane elements in a rack
Note 1: membrane “rack” (installation) looks like matrix K×M, where K is a number of rows and M is as number of membrane elements in a row (M = 5 or 6);
Note 2: There cannot be more than 6 membrane elements in a row! The number of installations (B) calculation is based on this.
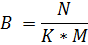
4.2.6. Specific capacity of single membrane element, m3/h
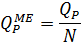
| QPME |
| QPME |
4.2.7. Calculation of QP:QC ratio for the last membrane element in a row:
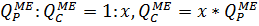
From here and below: QPME is the flow of the last membrane element in a row, m3/h. QCME is a transit flow of concentrate, m3/h.
This ratio (4.2.8.) is crucial for the last membrane element in a row, because there are the most difficult working conditions, in accordance with QP:QC ratio.
4.2.8. Calculation of flows for the whole row
The assumption: no pressure loss and no losses of flow in a row
| QPME |
| QCME = x*QPME QPME QPME QPME QPME |
| QFME = QPME+ QCME + QPME QPME QPME QPME |
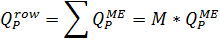
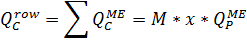
Where 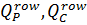 are flows of permeate and concentrate of a whole row of the membrane elements, respectively, m3/h;
are flows of permeate and concentrate of a whole row of the membrane elements, respectively, m3/h;
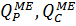 are flows of permeate and concentrate of one (last in a row) membrane element, respectively, m3/h;
are flows of permeate and concentrate of one (last in a row) membrane element, respectively, m3/h;
M is the number of membrane elements in a row;
x is the ratio between QPME and QCME;
Flows in a row are calculating from the last ME in a row to the first, therefore balance of flows fulfills for every single element as well as for the whole installation.
4.2.9. Flow balance for membrane installation
In one hand
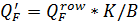
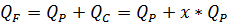
Where
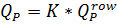
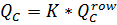
Then 
Qr is recycling flow, m3/h (See Figure XX.)
| QP |
| QC |
| QW |
| Qr |
| QF |
4.2.10. Concentration (Salt) balance
The main formulas are:
Salt concentration for permeate:
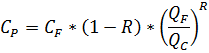
Salt concentration for concentrate:
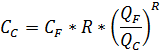
Principal calculations are similar to the flow balance.
After all calculations have been done, two graphic schemes are drown, showing all chains in the installations, depicting both flows and concentrations for every single membrane element as well as the whole rack.
4.3.The selection of pump for membrane installation
Problems
4.4.1. Problem 1
Find (calculate) the value of π for Black Sea water: salt concentration (as NaCl) – 32, 0 g/l; temperature – 20oС;
Molar mass (NaCl) – 58,5; R = 8,31; i =2
Solution: 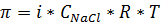 CNaCl = m/M
CNaCl = m/M
4.4.2. Problem 2
CF = 5 g/l
R = 99.6% = 0.996
Calculate the CP, if ratio QP:QC = 1:1
Solution:
QF = QP + QC =2; QF/QC = 2:1
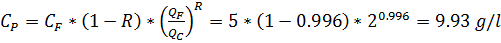
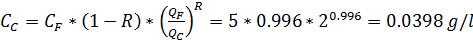
4.4.3. Problem 3
We have Dead Sea water with the initial salt concentration CF = 50 g/l.
If we use membrane with R-characteristic R = 99% and set the QP = 15% will we get freshwater?
Solution:
In potable water salt concentration, CS is ≤1000 mg/l, in freshwater CS ~ 200mg/l. Set the ratios: QF =1, QC = 0.85, QF/QC = 1.176
When
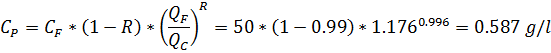
Answer: Yes, we will get water as potable, but not as freshwater quality
4.4.4. Problem 4
Membrane separation process of NaCl solution has the following parameters:
Filtrate flow Qf = 10 m3/h; Filtrate-concentrate ratio Qf:Qc = 1:2; Concentration of NaCl in a filtrate Cf = 1 mg/l
Membrane selectivity R = 98% (0,98)
Mass balance equation: Qf*Cf = Qp*Cp + Qc*Cc
Formula of salts concentration in a concentrate: 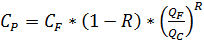
Formula of salts concentration in a filtrate: 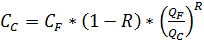
No concentrate flow is recycled
Find the values of feed flow, m3/h and NaCl concentration in the feed flow, mg/l
Solution:
4.4.5. Problem 5
Given: water hardness H = 5 mg-eq/l*, *mg-equivalent/l ≡ O
C (SO42-) = 500 mg/l
Find the value of k (flow concentrating coefficient), which cannot cause the formation of CaSO4 on the membrane surface.
Solution:
Concentration, CF (in Ca2+) = 5*20.04 (Ca equivalent) = 100.2 mg/l
When salt concentration: Ca2+ + SO42- = 100.2+500 = 600.2 mg/l
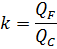
QF = m/CF, QC = m/CC,
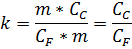
The limit of solubility for gypsum (that is CC actually) = 2000 mg/l,
When, in proportion:
CCaSO4 = 2000, M(CaSO4) = 136; M(Ca2+) = 40, CCa2+ =?
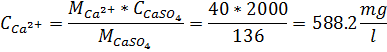
And 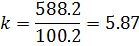
Answer: the k should be less than 5.87 for scaling mitigation
4.4.6. Problem 6
While carrying the desalination of Black Sea water (С0 = 32,0 g/l) the observing selectivity of polyamide membrane was R’ = 98.2%, despite of its nominal value (in the passport of installation) Rnom = 99,1%.
Find the Cm(st) – the concentration in the layer near membrane surface, g/l, in steady-state conditions
Solution:
Find CS(st) – the concentration in a solution in steady-state conditions:
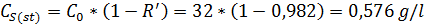
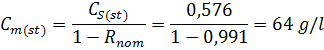
4.4.7. Problem 7
For sea water desalination (C0 = 32 g/l in NaCl) 4 membrane types are offered, with different salt retention ability (selectivity, R):
a) polypropylene membrane, R = 50%
b) polysulfone membrane, R = 90%
c) CA-membrane (cellulose acetate), R = 98,5%
d) polyamide membrane, R = 99,8%
Define the most suitable membrane for potable water production, if standard sets the CS = 500 mg/l in potable water.
Solution:
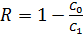
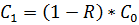 , where C1 – salt concentration after the desalination process;
, where C1 – salt concentration after the desalination process;
For a) 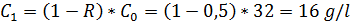
For b) 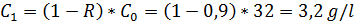
For c) 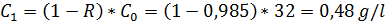
For d) 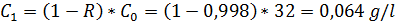
Answer membranes c) and d) are suitable for potable water production
4.4.8. Problem 8
Given:
Для опреснения воды Черного моря (С0 = 32 г/л) проектируется установка обратного осмоса производительностью по пермеату QP = 100 m3/h. Используются рулонные МЭ на полиамидной мембране, рассчитанные на рабочее давление 55 bar, с наблюдаемой селективностью по NaCl 98,2%. площадь мембраны в рулонном модуле = 3 м2. Удельная производительность этой мембраны в предварительных испытаниях, проведенных на водопроводной воде (0,5 г/л по NaCl), при рабочем давлении 15 атм, составила G = 55 л/м2ч.
Усредненная концентрация продукта не должна превышать 0,75 г/л. КПД насоса – 0,65 (65%), рекуператора энергии – 0,75 (75%). Гидравлическое сопротивление установки – 5 бар.
Рассчитать необходимую площадь мембран количество модулей.
Определить удельный расход энергии (кВтч/м3) без рекуперации и с рекуперацией.
Solution:
1) Из результатов предварительных испытаний определяем коэффициент удельной производительности мембран КG:
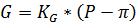 ,
, 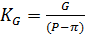
π – осмотическое давление водопроводной воды рассчитано ранее,
π = 0,4 бар = 4 ∙104 Па.
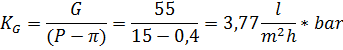
2) Концентрация пермеата на входе в установку составит
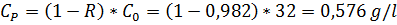
Рассматривая усредненную концентрацию продукта как среднее арифметическое между концентрациями пермеата на входе и на выходе, определим
Спвых = 2С – Спвх = 2∙0,75 – 0,576 = 0,924 г/л.
Концентрация концентрата (при условии R = const) равна
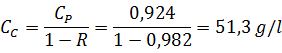
3) Mass balance of desalination process is

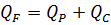
Отсюда определяем QC = 162 м3/ч и QF = 262 м3/ч.
Степень извлечения продукта (коэффициент конверсии)
Кконв = QP/QF = 100/262 = 0,38.
4) Среднее осмотическое давление в установке (из-за повышения концентрации
протекающего раствора) составит:
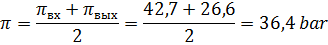
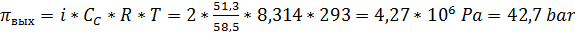
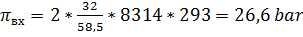
Среднее рабочее давление в установке 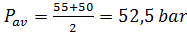
Удельная производительность мембран в этих условиях будет:
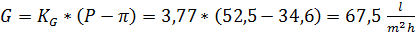
5) Необходимая площадь мембран в установке
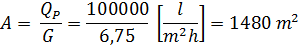
Потребное количество модулей:
N = F/Fмод = 1480/3 = 493 шт. ≈ 500 шт.
6) Расход энергии на нагнетание морской воды в опреснительную установку
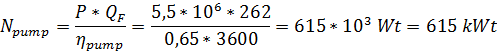
Возможная рекуперация энергии:
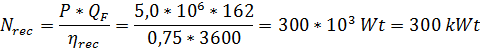
Удельный расход энергии без рекуперации:
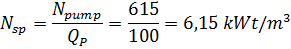
Удельный расход энергии с рекуперацией –
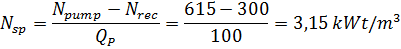
Конструктивно рекуператоры выполнены или в виде турбины, вырабатывающей электроэнергию, или совмещены с плунжерным нагнетательным насосом.
Дата добавления: 2020-04-12; просмотров: 601;











