Chemical Equilibrium
State the irreversible reactions in which the starting materials are fully converted into reaction products, i.e. reaction goes to completion. Signs of the irreversibility:
a) precipitation: Na2SO4 + BaCl2 → 2NaCl + BaSO4↓;
b) the allocation of gas: Na2CO3 + H2SO4 → Na2SO4 + H2O + CO2↑;
c) formation of a weak electrolyte: 2NaOH + H2SO4 → Na2SO4 + H2O.
State the reversible reactions in which the final products interact to form the starting materials.
Such reactions are not going to end, to a state of equilibrium.
Chemical equilibrium – a state of the system in which speed forward and reverse reactions are equal. Equilibrium is called concentration, which are installed on the equilibrium state (for the initial equilibrium concentration of the substance is the amount of material which remained at the time of equilibrium for the reaction products - is the amount of matter, which was formed at the time of equilibrium).
Chemical equilibrium is characterized by the equilibrium constant Кp, which is the ratio of product concentrations of the reaction products to the product of the concentrations of substances in the initial degrees are stoichiometric coefficients. In accordance with the law of mass action for the reversible reaction:
aA + bB → cC + dD
expression of the Кp can be written as follows:
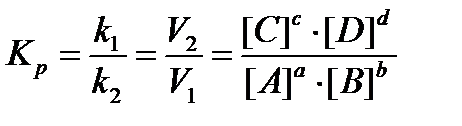
Thermodynamic equilibrium condition: ΔG = 0, ΔF = 0.
Кp shows how many times the rate of direct reaction greater than the rate of reverse reaction:
· If the Кр > 1, the faster the direct reaction; ΔG <0.
· If the Кр <1, then quickly goes back reaction; ΔG> 0.
· If Кр = 1, then ΔG = 0 (equilibrium state).
Кp depends on the nature of the reactants and temperature, and does not depend on the concentration of the catalyst.
Displacement of chemical equilibrium - is the transition system from one equilibrium state to another.
According to Le Chatelier's principle: if the system is in equilibrium, to produce effects (change the concentration, pressure, temperature), the equilibrium is shifted in the direction of the reaction, which weakens this effect.
This principle of Le Chatelier’s highlights the behavior of a system at equilibrium, if it is subjected to changes in parameters like pressure, temperature, or concentration.
Chemical equilibria can be shifted by changing the conditions that the system experiences. We say that we “stress” the equilibrium. When we stress the equilibrium, the chemical reaction is no longer at equilibrium, and the reaction starts to move back toward equilibrium in such a way as to decrease the stress. The formal statement is called Le Chatelier’s principle: If an equilibrium is stressed, then the reaction shifts to reduce the stress.
There are several ways to stress an equilibrium. One way is to add or remove a product or a reactant in a chemical reaction at equilibrium. When additional reactant is added, the equilibrium shifts to reduce this stress: it makes more product. When additional product is added, the equilibrium shifts to reactants to reduce the stress. If reactant or product is removed, the equilibrium shifts to make more reactant or product, respectively, to make up for the loss.
Example 1
Given this reaction at equilibrium: N2 + 3 H2 ⇄ 2 NH3
In which direction – toward reactants or toward products – does the reaction shift if the equilibrium is stressed by each change?
1) H2 is added.
2) NH3 is added.
3) NH3 is removed.
Solution:
If H2 is added, there is now more reactant, so the reaction will shift toward products to reduce the added H2.
If NH3 is added, there is now more product, so the reaction will shift toward reactants to reduce the added NH3.
If NH3 is removed, there is now less product, so the reaction will shift toward products to replace the product removed.
It is worth noting that when reactants or products are added or removed, the value of the Keq does not change. The chemical reaction simply shifts, in a predictable fashion, to reestablish concentrations so that the Keq expression reverts to the correct value.
How does an equilibrium react to a change in pressure? Pressure changes do not markedly affect the solid or liquid phases. However, pressure strongly impacts the gas phase. Le Chatelier’s principle implies that a pressure increase shifts an equilibrium to the side of the reaction with the fewer number of moles of gas, while a pressure decrease shifts an equilibrium to the side of the reaction with the greater number of moles of gas. If the number of moles of gas is the same on both sides of the reaction, pressure has no effect.
Example 2
What is the effect on this equilibrium if pressure is increased?
N2(g) + 3 H2(g) ⇄ 2 NH3(g)
Solution:
According to Le Chatelier’s principle, if pressure is increased, then the equilibrium shifts to the side with the fewer number of moles of gas. This particular reaction shows a total of 4 mol of gas as reactants and 2 mol of gas as products, so the reaction shifts toward the products side.
What is the effect of temperature changes on an equilibrium? It depends on whether the reaction is endothermic or exothermic. Recall that endothermic means that energy is absorbed by a chemical reaction, while exothermic means that energy is given off by the reaction. As such, energy can be thought of as a reactant or a product, respectively, of a reaction:
endothermic: energy + reactants → products
exothermic: reactants → products + energy
Because temperature is a measure of the energy of the system, increasing temperature can be thought of as adding energy. The reaction will react as if a reactant or a product is being added and will act accordingly by shifting to the other side. For example, if the temperature is increased for an endothermic reaction, essentially a reactant is being added, so the equilibrium shifts toward products. Decreasing the temperature is equivalent to decreasing a reactant (for endothermic reactions) or a product (for exothermic reactions), and the equilibrium shifts accordingly.
Example 3
Predict the effect of increasing the temperature on this equilibrium.
PCl3 + Cl2 ⇄ PCl5 + 60 kJ
Solution:
Because energy is listed as a product, it is being produced, so the reaction is exothermic. If the temperature is increasing, a product is being added to the equilibrium, so the equilibrium shifts to minimize the addition of extra product: it shifts back toward reactants.
In the case of temperature, the value of the equilibrium has changed because the Keq is dependent on temperature. That is why equilibria shift with changes in temperature.
A catalyst is a substance that increases the speed of a reaction. Overall, a catalyst is not a reactant and is not used up, but it still affects how fast a reaction proceeds. However, a catalyst does not affect the extent or position of a reaction at equilibrium. It helps a reaction achieve equilibrium faster.
Catalyst
A catalyst is a substance that speeds up the rate of a chemical reaction but is not consumed during the course of the reaction. A catalyst will appear in the steps of a reaction mechanism, but it will not appear in the overall chemical reaction (as it is not a reactant or product). Generally, catalysts alter the mechanism of the reaction in a substantial way such that the new barriers along the reaction coordinate are significantly lower. By lowering the activation energy, the rate constant is greatly increased (at the same temperature) relative to the uncatalyzed reaction.
There are many types of catalysts in the world. Many reactions are catalyzed at the surface of metals. In biochemistry, enormous numbers of reactions are catalyzed by enzymes. Catalysts can either be in the same phase as the chemical reactants or in a distinct phase.
Another important idea about catalysts is that they are selective. That is the catalyst doesn't just speed up all reactions, but only a very particular reaction. This is the key to many chemical transformations. When you only want to perform a particular chemical change, you look for a catalyst that will speed up that specific reaction but not others. Enzymes are remarkable in this way. Living biological systems require a myriad of specific chemical transformations and there is a unique enzyme to catalyze each of them.
Types of Catalysts. Catalysts can either be in the same phase as the chemical reactants or in a distinct phase.
Catalysts in the same phase are called homogeneous catalysts, while those in different phases are called heterogeneous catalysts.
For example, if we have Pt metal as a catalyst for the reaction of hydrogen gas and ethene gas, then the Pt is a heterogeneous catalyst. However, an enzyme in solution catalyzing a solution phase biochemical reaction is a homogeneous catalyst.
There are general characteristics of catalyst:
· A catalyst does not initiate the reaction. It simply fastens it.
· Only a small amount of catalyst can catalyse the reaction.
· A catalyst does not alter the position of equilibrium i.e. magnitude of equilibrium constant and hence ∆G0. It simply lowers the time needed to attain equilibrium. This means if a reversible reaction in absence of catalyst completes to go to the extent of 75% till attainment of equilibrium, and this state of equilibrium is attained in 20 minutes then in presence of a catalyst also the reaction will go to 75% of completion before the attainment of equilibrium but the time needed for this will be less than 20 minutes.
·A catalyst drives the reaction through a different route for which energy barrier is of shortest height and hence Ea is of lower magnitude. That is, the function of the catalyst is to lower down the activation.
References
1) http://chemistry.tutorvista.com/physical-chemistry/collision-theory.html
2) http://www.askiitians.com/iit-jee-chemistry/chemical-kinetics.aspx
3) Source: Boundless. “The Collision Theory.” Boundless Chemistry Boundless, 25 Aug. 2016. Retrieved 22 Feb. 2017 from https://www.boundless.com/chemistry/textbooks/boundless-chemistry-textbook/chemical-kinetics-13/activation-energy-and-temperature-dependence-100/the-collision-theory-422-7067/
Дата добавления: 2017-05-02; просмотров: 1413;











