Preparation of tablets
Amino acids
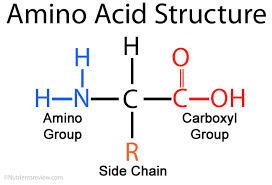
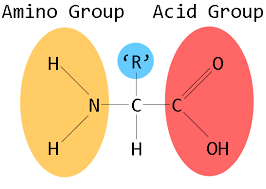
Amino acids are molecules containing an amine group, a carboxylic acid group, and a side-chain that is specific to each amino acid.
Amino acids serve as the building blocks of proteins, which are linear chains of amino acids. Amino acids can be linked together in varying sequences to form a vast variety of proteins. Twenty amino acids are naturally incorporated into polypeptides and are called proteinogenic or standard amino acids. These 20 are encoded by the universal genetic code. Nine standard amino acids are called "essential" for humans because they cannot be created from other compounds by the human body, and so must be taken in as food.
Amino acids are important in nutrition and are commonly used in nutrition supplements, fertilizers, food technology and industry.
Amino acids are the structural units that make up proteins. They join together to form short polymer chains called peptides or longer chains called either polypeptides or proteins. These polymers are linear and unbranched, with each amino acid within the chain attached to two neighboring amino acids. The process of making proteins is called translation and involves the step-by-step addition of amino acids to a growing protein chain by a ribozyme that is called a ribosome.
When taken up into the human body from the diet, the 22 standard amino acids either are used to synthesize proteins and other biomolecules or are oxidized to urea and carbon dioxide as a source of energy. The oxidation pathway starts with the removal of the amino group by a transaminase, the amino group is then fed into the urea cycle. Glycogenic amino acids can also be converted into glucose, through gluconeogenesis.
Of the 22 standard amino acids, 9 are called essential amino acids because the human body cannot synthesize them from other compounds at the level needed for normal growth, so they must be obtained from food. In addition, cysteine, taurine, tyrosine, and arginine are semi essential amino-acids in children, because the metabolic pathways that synthesize these amino acids are not fully developed. The amounts required also depend on the age and health of the individual, so it is hard to make general statements about the dietary requirement for some amino acids.
Carbohydrates
Carbohydrates in our foods are made by green plants. Leaves capture the sun’s heat and light in special areas of their cells and transform this to chemical energy. This energy then produces glucose from the carbon dioxide the leaves take from the air and the water the roots bring up from the soil. This complex process is called photosynthesis.
As the name suggests, most carbohydrates molecules are composed of carbon, hydrogen and oxygen atoms. Simple forms of carbohydrates are called sugars, while larger, more complex forms are called either starches or dietary fibers. Monosaccharides are single sugar forms. Glucose is the major monosaccharide found in the body. Glucose is also known as dextrose or blood sugar, since it is the major sugar in the blood stream. Fructose-also called laevulose or fruit sugar – is another common sugar. After it is consumed, fructose is absorbed by the small intestine and then transported to the liver.
The main function of glucose is to supply energy for the body. Certain tissues in the body, such as red blood cells, can use only glucose and other simple carbohydrate forms for energy. Most parts of the brain also derive energy only from simple carbohydrates. Simple carbohydrates can also fuel muscle cells and other body cells, but many of these also use fat for energy needs. Under normal circumstances, a person’s blood glucose level is regulated within a very narrow range. If blood glucose rises too high, the condition is called hyperglycemia (hyper- means high and -emia means in the bloodstream).
The liver is the first organ to screen the absorbed sugars. One of its roles is to guard against excess glucose entering the bloodstream after a meal. The pancreas works with the liver to control glucose levels.

Herbal medicine
Herbal medicine - also called botanical medicine or phytomedicine refers to using a plant's seeds, berries, roots, leaves, bark, or flowers for medicinal purposes. Herbalism has a long tradition of use outside of conventional medicine. It is becoming more mainstream as improvements in analysis and quality control along with advances in clinical research show the value of herbal medicine in the treating and preventing diseases.
Herbal medicine is used to treat many conditions, such as asthma, eczema, premenstrual syndrome, rheumatoid arthritis, migraine, menopausal symptoms, chronic fatigue, irritable bowel syndrome, and cancer, among others. Herbal supplements are best taken under the guidance of a trained health care provider. Be sure to consult with your doctor or pharmacist before taking any herbs. Some common herbs and their uses are discussed below.
Dandelions are one of the most multipurpose herbs. The leaves are eaten as a tasty spring green. The blossom is used to make jelly, fried in the bud stage as a delicious side dish. The delicate petals are added to salads and as a lovely edible flower addition to summer dishes. The root is also used, dried and ground as a substitute for coffee. As a medicinal plant, the blossom is added to oil and infused, to relieve aching muscles. The leaf and root are used in teas, tinctures, salves and oils as a liver tonic, soothing skin and muscle herb.
Calendula is an important addition to a healer's garden. Its striking orange flowers are used as a soothing skin wash, tea and salve. They are edible for a cheerful addition to a salad as well. Because it is so gentle, calendula is often an ingredient in diaper salves and other baby related skincare items. The flowers will readily reseed themselves, so consider this when planting.
Burdock is a common herb that is overlooked in American gardening. Its root used as a blood purifier and an overall medicinal vegetable. The leaf can be applied as a poultice to draw out infection. The seed is a much stronger medicine, and should be used with caution.
Chamomile is a sweetly scented, light tasting herb. Chamomile is a gentle soother for teas and skin washes. There has been some discussion about contraindications of this well known herb. As a disclaimer, if an individual has an allergy to ragweed, they may react to chamomile with the same symptoms. This is rare, but should be mentioned. Another effect that should be at least mentioned, is that there is some proof that chamomile is a blood thinner. This would not apply to an occasional cup of tea used as an evening treat, however, if someone was on blood thinning medication, they should bring their tea use to their medical provider's attention.


Solutions
A solution is a homogenous mixture of substances with variable composition. The substance present in the major proportion is called the solvent, whereas the substance present in the minor proportion is called the solute. It is possible to have solutions composed of several solutes. The process of a solute dissolving in a solute is called dissolution.
Many common mixtures (like concrete) are heterogeneous —the components and properties of such mixtures are not distributed uniformly throughout their structures. Conversely, solutions are said to be homogeneous because they have uniform composition and properties. Solutions are intimate and random homogeneous mixtures of atomic-size chemical species, ions, or molecules.
In addition to their observed homogeneity, true solutions also have certain other characteristics. For example, components of a solution never separate spontaneously, even when a significant density difference exists between the components. Solutions also pass through the finest filters unchanged.
The components of a solution distribute themselves in a completely random manner, given sufficient time. For example, a lump of sugar dropped into a glass of water dissolves, and eventually molecules of sugar can be found randomly distributed throughout the water, even though no mechanical stirring has been employed. This phenomenon, called diffusion, is similar to the process of diffusion that occurs with gases. The molecules of sugar (as well as those of water) must be in constant motion in the solution. In the case of liquid solutions, the sugar molecules do not move very far before they encounter other molecules; diffusion in a liquid is therefore less rapid than diffusion in gas.
Kinds of solutions.Many commonly encountered solutions are those involving a solid that has been dissolved in a liquid, but there are as many types of solutions as there are different combinations of solids, liquids, and gases. Solutions in which the solvent is a liquid and the solute is a gas, liquid, or solid are very common. The atmosphere is a good example of a solution in which a gaseous solvent (nitrogen) dissolves other gases (such as oxygen, carbon dioxide, water vapor, and neon). Solutions of solids in solids are another example, and these are encountered most often among the various metal alloys.
Of all the liquid solvents used in the laboratory, in industry, and in the home, water is the most commonly employed and is the best of the inorganic solvents. The alcohols and numerous other types of compounds are classified as organic solvents; many of these are used in dry cleaning chemicals, nail polish removers, paint thinners, and many other similar purposes.


Preparation of tablets
The chemists say that the tablet is the most common form of medication for the administering of drugs in a dry state. Its preparation constitutes an important part of modern “Pharmaceutical Technology”.
From a purely physical point of view, the technique of tablet making or tableting may be defined as a process whereby a known volume of a drug in a finely divided state is subjected to pressure in a die between two punches.
A tablet shows definite properties of mechanical strength and is also characterized by a definite rate of disintegration when brought into contact with water.
It is generally observed that tablets can be made easily from certain drugs, such as sodium chloride and other alkali halides, even without the addition of auxiliary substances. For some other drugs, as lactose, the addition of auxiliary substances is found to be necessary to overcome certain difficulties in their tableting. The chemists proved that some difficulties were occasionally experienced in the process of tableting certain materials because of persistent binding or sticking in the tablet machine.
Sticking may occur when there is too much moisture in the granules which in turn may be due to insufficient drying, etc.
The application of different pressure during tableting plays a very important role. The correct pressure must be applied in order avoid unnecessary complications. Tablets which should dissolve slowly in the mouth must be more strongly compressed than other average tablets for internal administration.
Another important effect of higher pressures is an increase in friction which obviously necessitates the use of greater amounts of lubricants and glidants. Glidants are added to the tablet materials to improve their flow properties. They are generally powdery substances which deform only slightly when subjected to the compressing pressures. To glidants belong such substances as natural starch, which has excellent flow improvement properties.
Lubricants are substances which facilitate smooth election of the compressed tablets.
The chemists said that the use of starch as an auxiliary in tablet making had been recognized for a very long time. It was stated that starches possess very good glidant properties in increasing the flow of tablet materials and they do not show any lubricating action.
Дата добавления: 2016-09-06; просмотров: 2496;











