Antigen-Derived Peptides Are the Key Elements of T-Cell Epitopes
Studies by P. G. H. Gell and Baruj Benacerraf in 1959 suggested that there was a qualitative difference between the T cell and the B-cell response to protein antigens. Gell and Benacerraf compared the humoral (B-cell) and cell-mediated (T-cell) responses to a series of native and denatured protein antigens. They found that when primary immunization was with a native protein, only native protein, not denatured protein, could elicit a secondary antibody (humoral) response. In contrast, both native and denatured protein could elicit a secondary cell-mediated response. The finding that a secondary response mediated by T cells was induced by denatured protein, even when the primary immunization had been with native protein, initially puzzled immunologists. In the 1980s, however, it became clear that T cells do not recognize soluble native antigen but rather recognize antigen that has been processed into antigenic peptides, which are presented in combination with MHC molecules. For this reason, destruction of the conformation of a protein by denaturation does not affect its T-cell epitopes.
Because the T-cell receptor does not bind free peptides, experimental systems for studying T-cell epitopes must include antigen-presenting cells or target cells that can display the peptides bound to an MHC molecule.
Antigenic peptides recognized by T cells form trimolecular complexes with a T-cell receptor and an MHC molecule (Figure4). The structures of TCR-peptide-MHC trimolecularcomplexes have been determined by x-ray crystallography. These structural studies ofclass I or class II MHC molecules crystallized with known T cellantigenic peptides has shown that the peptide binds to a epitopes,which can be viewed strictly in terms of their abilityto interact with antibody, T-cell epitopes must be viewed interms of their ability to interact with both a T-cell receptorand an MHC molecule.
The binding of an MHC molecule to an antigenic peptide does not have the fine specificity of the interaction between an antibody and its epitope. Instead, a given MHC molecule can selectively bind a variety of different peptides. For example, the class II MHC molecule designated IAd can bind peptides from ovalbumin (residues 323–339), hemagglutinin (residues 130–142), and lambda repressor (residues 12–26).
Antigen processing is required to generate peptides that interact specifically with MHC molecules. Endogenous and exogenous antigens are usuallyprocessed by different intracellular pathways. Endogenous antigens are processed into peptideswithin the cytoplasm, while exogenous antigens areprocessed by the endocytic pathway.
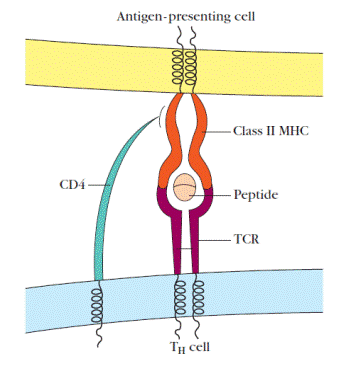
Figure 4. Schematic diagram of the ternary complex formed between a T-cell receptor (TCR) on a TH cell, an antigen, and a class II MHC molecule. Antigens that are recognized by T cells yield peptides that interact with MHC molecules to form a peptide-MHC complex that is recognized by the T-cell receptor. The coreceptor, CD4, on TH cells also interacts with MHC molecules. TC cells form similar ternary complexes with class I MHC molecules on target cells however, these cells bear MHC-interacting CD8 coreceptors.
Epitopes recognized by T cells are often internal. T cells tend to recognize internal peptides that are exposed by processing within antigen-presenting cells or altered self-cells. J. Rothbard analyzed the tertiary conformation of hen egg-white lysozyme and sperm whale myoglobin to determine which amino acids protruded from the natural molecule. He then mapped the major T-cell epitopes for both proteins and found that, in each case, the T-cell epitopes tended to be on the “inside” of the protein molecule.
Дата добавления: 2016-07-18; просмотров: 2502;











