The Biological System Contributes to Immunogenicity
Even if a macromolecule has the properties that contribute to immunogenicity, its ability to induce an immune response will depend on certain properties of the biological system that the antigen encounters. These properties include the genotype of the recipient, the dose and route of antigen administration, and the administration of substances, called adjuvants, that increase immune responses.
GENOTYPE OF THE RECIPIENT ANIMAL
The genetic constitution (genotype)of an immunized animal influences the type of immune response the animal manifests, as well as the degree of the response. For example, Hugh McDevitt showed that two different inbred strains of mice responded very differently to a synthetic polypeptide immunogen. After exposure to the immunogen, one strain produced high levels of serum antibody, whereas the other strain produced low levels. When the two strains were crossed, the F1 generation showed an intermediate response to the immunogen. By backcross analysis, the gene controlling immune responsiveness was mapped to a subregion of the major histocompatibility complex (MHC). Numerous experiments with simple defined immunogens have demonstrated genetic control of immune responsiveness, largely confined to genes within the MHC. These data indicate that MHC gene products, which function to present processed antigen to T cells, play a central role in determining the degree to which an animal responds to an immunogen.
The response of an animal to an antigen is also influenced by the genes that encode B-cell and T-cell receptors and by genes that encode various proteins involved in immune regulatory mechanisms. Genetic variability in all of these genes affects the immunogenicity of a given macromolecule in different animals.
IMMUNOGEN DOSAGE AND ROUTE OF ADMINISTRATION
Each experimental immunogen exhibits a particular dose-response curve, which is determined by measuring the immune response to different doses and different administration routes. An antibody response is measured by determining the level of antibody present in the serum of immunized animals. Evaluating T-cell responses is less simple but may be determined by evaluating the increase in the number of T cells bearing TCRs that recognize the immunogen. Some combination of optimal dosage and route of administration will induce a peak immune response in a given animal.
An insufficient dose will not stimulate an immune response either because it fails to activate enough lymphocytes or because, in some cases, certain ranges of low doses can induce a state of immunologic unresponsiveness, or tolerance. Conversely, an excessively high dose can also induce tolerance. The immune response of mice to the purified pneumococcal capsular polysaccharide illustrates the importance of dose. A 0.5 mg dose of antigen fails to induce an immune response in mice, whereas a thousand-fold lower dose of the same antigen (5 × 10-4 mg) induces a humoral antibody response. A single dose of most experimental immunogens will not induce a strong response; rather, repeated administration over a period of weeks is usually required. Such repeated administrations, or boosters,increase the clonal proliferation of antigen-specific T cells or B cells and thus increase the lymphocyte populations specific for the immunogen.
Experimental immunogens are generally administered parenterally (para, around; enteric, gut) – that is, by routes other than the digestive tract. The following administration routes are common:
- Intravenous (iv): into a vein
- Intradermal (id): into the skin
- Subcutaneous (sc): beneath the skin
- Intramuscular (im): into a muscle
- Intraperitoneal (ip): into the peritoneal cavity
The administration route strongly influences which immune organs and cell populations will be involved in the response. Antigen administered intravenously is carried first to the spleen, whereas antigen administered subcutaneously moves first to local lymph nodes. Differences in the lymphoid cells that populate these organs may be reflected in the subsequent immune response.
ADJUVANTS
Adjuvants(from Latin adjuvare, to help) are substances that, when mixed with an antigen and injected with it, enhance the immunogenicity of that antigen. Adjuvants are often used to boost the immune response when an antigen has low immunogenicity or when only small amounts of an antigen are available. For example, the antibody response of mice to immunization with BSA can be increased fivefold or more if the BSA is administered with an adjuvant. Precisely how adjuvants augment the immune response is not entirely known, but they appear to exert one or more of the following effects:
- Antigen persistence is prolonged.
- Co-stimulatory signals are enhanced.
- Local inflammation is increased.
- The nonspecific proliferation of lymphocytes is stimulated.
Aluminum potassium sulfate (alum) prolongs the persistence of antigen. When an antigen is mixed with alum, the salt precipitates the antigen. Injection of this alum precipitate results in a slower release of antigen from the injection site, so that the effective time of exposure to the antigen increases from a few days without adjuvant to several weeks with the adjuvant. The alum precipitate also increases the size of the antigen, thus increasing the likelihood of phagocytosis.
Water-in-oil adjuvants also prolong the persistence of antigen. A preparation known as Freund’s incomplete adjuvant contains antigen in aqueous solution, mineral oil, and an emulsifying agent such as mannide monooleate, which disperses the oil into small droplets surrounding the antigen; the antigen is then released very slowly from the site of injection. This preparation is based on Freund’s complete adjuvant,the first deliberately formulated highly effective adjuvant, developed by Jules Freund many years ago and containing heat-killed Mycobacteria as an additional ingredient. Muramyl dipeptide, a component of the mycobacterial cell wall, activates macrophages, making Freund’s complete adjuvant far more potent than the incomplete form. Activated macrophages are more phagocytic than unactivated macrophages and express higher levels of class II MHC molecules and the membrane molecules of the B7 family. The increased expression of class II MHC increases the ability of the antigen-presenting cell to present antigen to TH cells. B7 molecules on the antigenpresenting cell bind to CD28, a cell-surface protein on TH cells, triggering co-stimulation, an enhancement of the Tcell immune response. Thus, antigen presentation and the requisite co-stimulatory signal usually are increased in the presence of adjuvant.
Alum and Freund’s adjuvants also stimulate a local, chronic inflammatory response that attracts both phagocytes and lymphocytes. This infiltration of cells at the site of the adjuvant injection often results in formation of a dense, macrophage-rich mass of cells called a granuloma.Because the macrophages in a granuloma are activated, this mechanism also enhances the activation of TH cells.
Other adjuvants (e.g., synthetic polyribonucleotides and bacterial lipopolysaccharides) stimulate the nonspecific proliferation of lymphocytes and thus increase the likelihood of antigen-induced clonal selection of lymphocytes.
Epitopes
Immune cells do not interact with, or recognize, an entire immunogen molecule; instead, lymphocytes recognize discrete sites on the macromolecule called epitopes,or antigenic determinants.Epitopes are the immunologically active regions of an immunogen that bind to antigen-specific membrane receptors on lymphocytes or to secreted antibodies. Studies with small antigens have revealed that B and T cells recognize different epitopes on the same antigenic molecule. For example, when mice were immunized with glucagon, a small human hormone of 29 amino acids, antibody was elicited to epitopes in the aminoterminal portion, whereas the T cells responded only to epitopes in the carboxyl-terminal portion.
Lymphocytes may interact with a complex antigen on several levels of antigen structure. An epitope on a protein antigen may involve elements of the primary, secondary, tertiary, and even quaternary structure of the protein. In polysaccharides, branched chains are commonly present, and multiple branches may contribute to the conformation of epitopes.
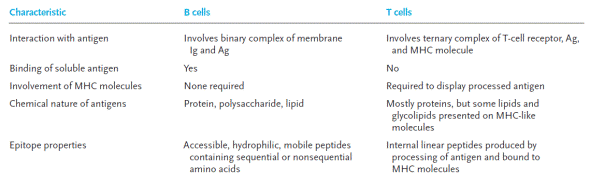
The recognition of antigens by T cells and B cells is fundamentally different (Table 1). B cells recognize soluble antigen when it binds to their membrane-bound antibody. Because B cells bind antigen that is free in solution, the epitopes they recognize tend to be highly accessible sites on the exposed surface of the immunogen. As noted previously, most T cells recognize only peptides combined with MHC molecules on the surface of antigen-presenting cells and altered self-cells; T-cell epitopes, as a rule, cannot be considered apart from their associated MHC molecules.
Table 1
Comparison of antigen recognition by T cells and B cells
Дата добавления: 2016-07-18; просмотров: 5236;











