Fig. 1.6. DNA structure
A strong chemical bond forms between the phosphate group of one nucleotide and the deoxyribose sugar of another. These bonds are not easily broken and join neighboring nucleotide units into a permanent strand. Two of these strands become joined together by weaker hydrogen bonds formed between these bases. However this union is temporary in those hydrogen bonds and can be easily broken when it becomes necessary. Each base can join with only one of the other types of the base: A always bonds with T, and G always bonds with C. A-T and G-C is called base pairs. Each member of a pair is complementary to its partner. The pairs of nucleotides forming the DNA ladder can appear in any order. The sequence of the nucleotides is the code that controls the production of all the proteins of an organism.
The resultant double stranded molecule is DNA and its two strands are arranged like a twisted ladder or coil. This twisted coil is called a double helix. The sugar and phosphate groups make up the sides of the ladder. The bases make up the rungs.
Chemically, RNA is very similar to DNA, but there are two differences in its chemical groups. One difference is in the sugar component; instead of deoxyribose, RNA contains ribose, which has an additional oxygen atom. The other difference is in nitrogen base - instead of thymine, RNA contains the closely related uracil (Fig. 1.7). The third, and very important, difference between the two is that most RNA does not possess a regular helical structure and is usually single-stranded (Fig. 1.8). Unlike DNA which is found primarily in the nucleus, most of the RNA is found in the cytoplasm and it is here that protein manufacture takes place.
Three types of RNA are involved: messenger RNA, transfer RNA and ribosomal RNA.
Cell making large amounts of protein have numerous ribosomes, and ribosomes are rich in RNA.
| phosphate ribose sugar | 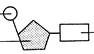
| Base | Fig. 1.7. Structure of RNA nucleotide |
Many molecules of adenosine triphosphate (ATP) are present in every living cell. ATP is composed of adenosine, ribose and three inorganic phosphate groups. ATP is able to immediately provide the energy required for muscle contraction whereas glucose, despite being an energy-rich compound, is unable to do so. ATP is also regenerated and used during photosynthesis.
Carbohydratesare molecules that supply much of the cell’s energy. The smallest carbohydrate molecules are sugars. Many sugars are joined together to form large starch molecules.
Cellulose is the principal structural polysaccharide of plants. It is formed within a plant cell and deposited outside of the cell membrane. Like starch and glycogen, cellulose is composed of units of glucose, but because of slight differences in molecular configuration, cellulose cannot be broken down by the enzymes that hydrolyze the storage polysaccharides.
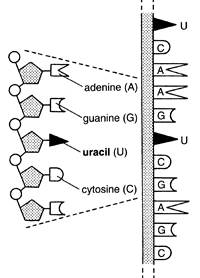
| |
| Fig. 1.8. Structure of RNA |
One of the earliest conclusions from biology as a modern experimental science was that even the tiniest microbe comes from others of the same type—that is, that life begets life. The conditions on primeval Earth that may have enabled life to arise from inanimate self-replicating chemicals no longer exist. Today all life comes from pre-existing life.
Дата добавления: 2016-07-18; просмотров: 1889;











