MATTER AND ITS STATES
Among the most important effects of heat is that of changing the state of matter from a solid to a liquid, from a liquid to a gas, from a gas to a plasma. In effect, some substances are capable of existing in each of the four possible states under suitable conditions of temperature and pressure. It is obvious that the process under consideration also depends on the quality of the substance as well as on its volume. In order to effect a change of state under ordinary atmospheric pressure, it is necessary either to add or to remove a certain definite quantity of heat. On adding heat, one may expect a solid to change into a liquid, the latter being turned, into, a gas. In some cases a solid body may change directly into a gas. Gas, in its turn, may be heated to a plasma state.
We generally find that each substance exists mainly in one given state. Iron, for instance, is usually thought of as a solid body, water as a liquid, and air as a gas. Nevertheless, we are also familiar with the transformation of the same kind of matter from its usual state to another and that transformation is effected by supplying or decreasing heat. For example, we know water to exist in three possible states, namely: as solid ice which can melt to form the liquid that we call "water," water in its turn evaporates to form a gas, that is to say, first vapour and then steam, when heated to the boiling point. The reader is unlikely to distinguish between the English terms "steam" and "vapour." As long as there is still some water left unevaporated in the container, the steam formed will not be pure steam, but will have some particles of water in suspension. Such steam is said to be wet steam and one may classify it as a vapour.
If we take a certain quantity of ice below the freezing point, that is below 0°C, and gradually heat it at a uniform rate, the temperature may be observed to rise steadily until the freezing point is reached. At this point the temperature stops rising and remains unchangeable while melting takes place. A considerable amount of heat is absorbed in order to effect the change of state from solid ice to water, while the temperature remains steady. This heat is said to be latent.
The ice having melted, the water again rises steadily in temperature until it begins to boil, turning rapidly into steam or water-gas. Then, again there is no rise in temperature and an even larger amount of "latent" heat is required but to effect the transformation from water to steam, without rise of temperature. Besides this rapid change at boiling, one may observe as well a gradual change into steam, even at ordinary temperatures.
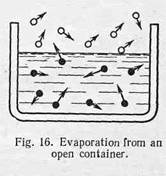
|
The process in question takes plaсе at the surface when water is in an open container, or any other open place. It follows that in the open there will be a constant loss from the surface of the liquid and this loss will increase as the temperature rises. The above phenomenon is known as evaporation.
Fig. 16 illustrates water evaporating from an open container. The black dots represent greatly enlarged water molecules, the circles representing air molecules, also enlarged. The small arrows indicate that both air and water molecules are in a continuous motion. It is only the rapidly-moving molecules which are able to leave the surface of the liquid. Therefore, the mean velocity of those left behind will be decreased, that is to say, the liquid will be cooled.
The reader probably remembers that evaporation may even take place from the surface of ice. This is the fact which is familiar to us because we see it in the disappearance of snow in a dry east wind, though the temperature does not rise to the melting point. Evaporation also consumes heat, a fact which may be easily illustrated as follows: if you wet one hand before going out on a cold day in winter, you will feel that your wet hand is much colder than the other one. It is the absorption of heat from your hand that causes the cooling effect.
It is necessary to point out that the same number and kind of molecules that are to be found in, say, a kilogram of steam are also present in that very amount of water or ice. Why, then, do these different states exist and why have they such widely varying properties? For the simple reason that molecules move differently in each of the states under consideration.
Exercises
1.Learn the following active words:
absorb впитывать, поглощать
effect осуществлять
evaporation испарение
suspension взвесь
latent heat скрытое тепло
pure чистый
state состояние
surface поверхность
rapid быстрый
2. Translate the following sentences paying attention to the participle:
1. Many substances can exist in more than one of the four possible states, that state depending on the substance itself as well as on its volume, temperature, and pressure. 2. On adding heat a solid may be changed into a liquid, the latter being changed into a gas. 3. Efficiency may be generally defined as output divided by input. 4. There is always water vapour in the air, the amount depending upon various conditions. 5. The resistance having been very high, the current in the circuit was low. 6. Steam is a gas into which boiling water changes, vapour consisting of the pure gaseous state together with particles of the liquid in suspension. 7. The flow of the current being reduced, the speed of the motor is decreased.
3. Answer the following questions:
1. What are the three states of matter? 2. Under what conditions does the change of a substance take place 3. When is a solid changed into a liquid? 4. What substance exist in the three possible states of matter? 5. What is the freezing point of water? 6. What does the term "latent heat' mean? 7. What do we call evaporation? 8. What is the boiling point of water? 9. When does the liquid boil?
4. Define the meaning of the following words:
boiling, evaporation, freezing, output, input
Дата добавления: 2016-07-27; просмотров: 2035;











