Secondary Lymphoid Organs
Various types of organized lymphoid tissues are located along the vessels of the lymphatic system. Some lymphoid tissue in the lung and lamina propria of the intestinal wall consists of diffuse collections of lymphocytes and macrophages. Other lymphoid tissue is organized into structures called lymphoid follicles, which consist of aggregates of lymphoid and nonlymphoid cells surrounded by a network of draining lymphatic capillaries. Until it is activated by antigen, a lymphoid follicle – called a primary follicle – comprises a network of follicular dendritic cells and small resting B cells. After an antigenic challenge, a primary follicle becomes a larger secondary follicle – a ring of concentrically packed B lymphocytes surrounding a center (the germinal center) in which one finds a focus of proliferating B lymphocytes and an area that contains nondividing B cells, and some helper T cells interspersed with macrophages and follicular dendritic cells.
Most antigen-activated B cells divide and differentiate into antibody-producing plasma cells in lymphoid follicles, but only a few B cells in the antigen-activated population find their way into germinal centers. Those that do undergo one or more rounds of cell division, during which the genes that encode their antibodies mutate at an unusually high rate. Following the period of division and mutation, there is a rigorous selection process in which more than 90% of these B cells die by apoptosis. In general, those B cells producing antibodies that bind antigen more strongly have a much better chance of surviving than do their weaker companions. The small number of B cells that survive the germinal center’s rigorous selection differentiate into plasma cells or memory cells and emerge.
Lymph nodesand the spleenare the most highly organized of the secondary lymphoid organs; they comprise not only lymphoid follicles, but additional distinct regions of T-cell and B-cell activity, and they are surrounded by a fibrous capsule. Less-organized lymphoid tissue, collectively called mucosal-associated lymphoid tissue (MALT), is found in various body sites. MALT includes Peyer’s patches (in the small intestine), the tonsils, and the appendix, as well as numerous lymphoid follicles within the lamina propria of the intestines and in the mucous membranes lining the upper airways, bronchi, and genital tract.
LYMPH NODES
Lymph nodes are the sites where immune responses are mounted to antigens in lymph. They are encapsulated beanshaped structures containing a reticular network packed with lymphocytes, macrophages, and dendritic cells. Clustered at junctions of the lymphatic vessels, lymph nodes are the first organized lymphoid structure to encounter antigens that enter the tissue spaces. As lymph percolates through a node, any particulate antigen that is brought in with the lymph will be trapped by the cellular network of phagocytic cells and dendritic cells (follicular and interdigitating). The overall architecture of a lymph node supports an ideal microenvironment for lymphocytes to effectively encounter and respond to trapped antigens.
Morphologically, a lymph node can be divided into three roughly concentric regions: the cortex, the paracortex, and the medulla, each of which supports a distinct microenvironment (Figure 5). The outermost layer, the cortex,contains lymphocytes (mostly B cells), macro-phages, and follicular dendritic cells arranged in primary follicles. After antigenic challenge, the primary follicles enlarge into secondary follicles, each containing a germinal center. In children with B-cell deficiencies, the cortex lacks primary follicles and germinal centers. Beneath the cortex is the paracortex,which is populated largely by T lymphocytes and also contains interdigitating dendritic cells thought to have migrated from tissues to the node. These interdigitating dendritic cells express high levels of class II MHC molecules,which are necessary for presenting antigen to TH cells. Lymph nodes taken from neonatally thymectomized mice have unusually few cells in the paracortical region; the paracortex is therefore sometimes referred to as a thymus-dependent areain contrast to the cortex, which is a thymus-independent area.The innermost layer of a lymph node, the medulla,is more sparsely populated with lymphoid-lineage cells; of those present, many are plasma cells actively secreting antibody molecules.
As antigen is carried into a regional node by the lymph, it is trapped, processed, and presented together with class II MHC molecules by interdigitating dendritic cells in the paracortex, resulting in the activation of TH cells. The initial activation of B cells is also thought to take place within the T-cell-rich paracortex. Once activated, TH and B cells form small foci consisting largely of proliferating B cells at the edges of the paracortex. Some B cells within the foci differentiate into plasma cells secreting IgM and IgG. These foci reach maximum size within 4–6 days of antigen challenge. Within 4–7 days of antigen challenge, a few B cells and TH cells migrate to the primary follicles of the cortex. It is not known what causes this migration.Within a primary follicle, cellular interactions between follicular dendritic cells, B cells, and TH cells take place, leading to development of a secondary follicle with a central germinal center. Some of the plasma cells generated in the germinal center move to the medullary areas of the lymph node, and many migrate to bone marrow.
Afferent lymphatic vessels pierce the capsule of a lymph node at numerous sites and empty lymph into the subcapsular sinus (see Figure 5b). Lymph coming from the tissues percolates slowly inward through the cortex, paracortex, and medulla, allowing phagocytic cells and dendritic cells to trap any bacteria or particulate material (e.g., antigen-antibody complexes) carried by the lymph. After infection or the introduction of other antigens into the body, the lymph leaving a node through its single efferent lymphatic vessel is enriched with antibodies newly secreted by medullary plasma cells and also has a fiftyfold higher concentration of lymphocytes than the afferent lymph.
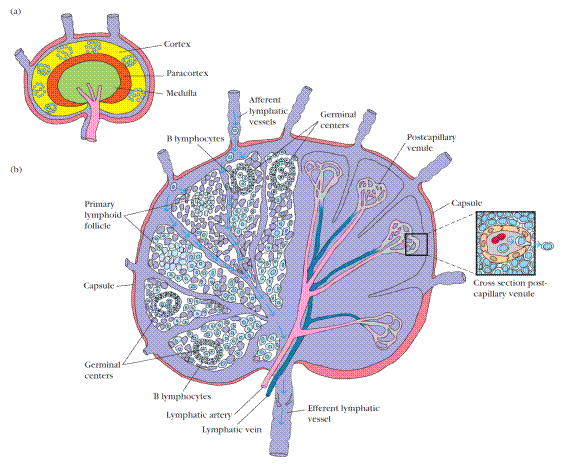
Figure 5. Structure of a lymph node. (a) The three layers of a lymph node support distinct microenvironments. (b) The left side depicts the arrangement of reticulum and lymphocytes within the various regions of a lymph node. Macrophages and dendritic cells, which trap antigen, are present in the cortex and paracortex. TH cells are concentrated in the paracortex; B cells are located primarily in the cortex, within follicles and germinal centers. The medulla is populated largely by antibody-producing plasma cells. Lymphocytes circulating in the lymph are carried into the node by afferent lymphatic vessels; they either enter the reticular matrix of the node or pass through it and leave by the efferent lymphatic vessel. The right side of (b) depicts the lymphatic artery and vein and the postcapillary venules. Lymphocytes in the circulation can pass into the node from the postcapillary venules by a process called extravasation (inset).
The increase in lymphocytes in lymph leaving a node is due in part to lymphocyte proliferation within the node in response to antigen. Most of the increase, however, represents blood-borne lymphocytes that migrate into the node by passing between specialized endothelial cells that line the postcapillary venulesof the node. Estimates are that 25% of the lymphocytes leaving a lymph node have migrated across this endothelial layer and entered the node from the blood. Because antigenic stimulation within a node can increase this migration tenfold, the concentration of lymphocytes in a node that is actively responding can increase greatly, and the node swells visibly. Factors released in lymph nodes during antigen stimulation are thought to facilitate this increased migration.
SPLEEN
The spleen plays a major role in mounting immune responses to antigens in the blood stream. It is a large, ovoid secondary lymphoid organ situated high in the left abdominal cavity. While lymph nodes are specialized for trapping antigen from local tissues, the spleen specializes in filtering blood and trapping blood-borne antigens; thus, it can respond to systemic infections. Unlike the lymph nodes, the spleen is not supplied by lymphatic vessels. Instead, bloodborne antigens and lymphocytes are carried into the spleen through the splenic artery. Experiments with radioactively labeled lymphocytes show that more recirculating lymphocytes pass daily through the spleen than through all the lymph nodes combined.
The spleen is surrounded by a capsule that extends a number of projections (trabeculae) into the interior to form a compartmentalized structure. The compartments are of two types, the red pulp and white pulp, which are separated by a diffuse marginal zone (Figure 6). The splenic red pulp consists of a network of sinusoids populated by macrophages and numerous red blood cells (erythrocytes) and few lymphocytes; it is the site where old and defective red blood cells are destroyed and removed. Many of the macrophages within the red pulp contain engulfed red blood cells or iron pigments from degraded hemoglobin. The splenic white pulpsurrounds the branches of the splenic artery, forming a periarteriolar lymphoid sheath (PALS)populated mainly by T lymphocytes. Primary lymphoid follicles are attached to the PALS. These follicles are rich in B cells and some of them contain germinal centers. The marginal zone,located peripheral to the PALS, is populated by lymphocytes and macrophages.
Blood-borne antigens and lymphocytes enter the spleen through the splenic artery, which empties into the marginal zone. In the marginal zone, antigen is trapped by interdigitating dendritic cells, which carry it to the PALS. Lymphocytes in the blood also enter sinuses in the marginal zone and migrate to the PALS.
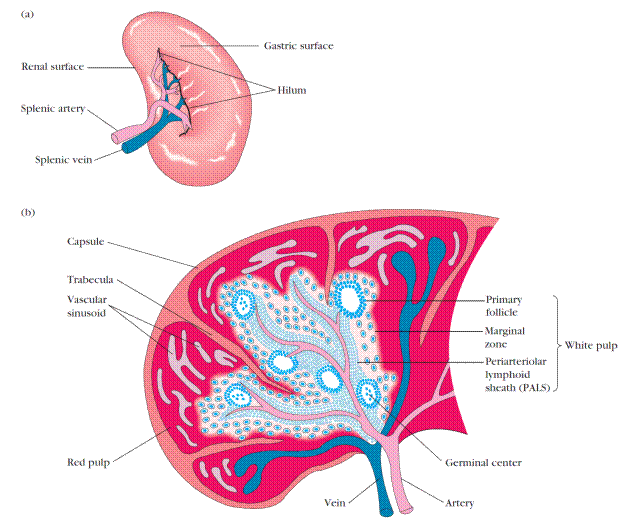
Figure 6. Structure of the spleen. (a) The spleen, which is about 5 inches long in adults, is the largest secondary lymphoid organ. It is specialized for trapping blood-borne antigens. (b) Diagrammatic cross section of the spleen. The splenic artery pierces the capsule and divides into progressively smaller arterioles, ending in vascular sinusoids that drain back into the splenic vein. The erythrocyte-filled red pulp surrounds the sinusoids. The white pulp forms a sleeve, the periarteriolar lymphoid sheath (PALS), around the arterioles; this sheath contains numerous T cells. Closely associated with the PALS is the marginal zone, an area rich in B cells that contains lymphoid follicles that can develop into secondary follicles containing germinal centers.
The initial activation of B and T cells takes place in the Tcell-rich PALS. Here interdigitating dendritic cells capture antigen and present it combined with class II MHC molecules to TH cells. Once activated, these TH cells can then activate B cells. The activated B cells, together with some TH cells, then migrate to primary follicles in the marginal zone. Upon antigenic challenge, these primary follicles develop into characteristic secondary follicles containing germinal centers (like those in the lymph nodes), where rapidly dividing B cells (centroblasts) and plasma cells are surrounded by dense clusters of concentrically arranged lymphocytes.
The effects of splenectomy on the immune response depends on the age at which the spleen is removed. In children, splenectomy often leads to an increased incidence of bacterial sepsis caused primarily by Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae. Splenectomy in adults has less adverse effects, although it leads to some increase in blood-borne bacterial infections (bacteremia).
Дата добавления: 2016-07-18; просмотров: 4470;











